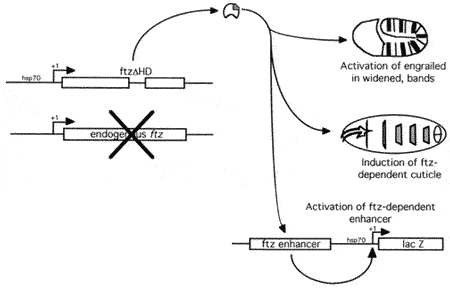
Role of FTZ in determining the number of segments
What we have learned so far.
When I was a postdoctoral fellow in Henry Krause's laboratory, we found that a Fushi tarazu (FTZ) protein that lacked the homeodomain was still active. This result was surprising because the homeodomain is the DNA-binding domain of FTZ and FTZ has the properties of a DNA-binding transcription factor. Hence, how does FTZ, a DNA-binding transcription factor, have activity without being able to bind DNA itself? A part of the solution to this problem came from an international collaboration that we were involved in (Guichet et al., 1997). The FTZ that lacks the homeodomain can still interact with DNA indirectly by binding a DNA-binding, FTZ cofactor FTZ-F1.

However, this is not a complete solution. Denis Hyduk showed that FTZ lacking the homeodomain was able to perform most activities associated with FTZ (Hyduk and Percival-Smith, 1996). One of the most interesting observations made previously and observed again in Denis's experiments was that activation of the FTZ-dependent regulatory element the ftz enhancer was spatially restricted even when FTZ protein was expressed in all cells. Also the activation of the ftz enhancer by a FTZ molecule that lacked the homeodomain required active homeodomain DNA binding sites in the ftz enhancer. We hypothesize the presence of a second FTZ cofactor that is spatially localized and recognizes homeodomain DNA binding sites.
Where we are going.
We initiated a systematic deletion analysis of FTZ to determine what was required for FTZ activity. We found that the FTZ-F1 interaction site was required for FTZ activity (Guichet et al., 1997). We are now determining what is sufficient for FTZ activity. This is a more difficult analysis because we have found that the N and C termini of FTZ are functionally redundant. We are presently testing what mediates this functional redundancy. Since FTZ activity requires a FTZ-F1 binding site and something else, it is tempting to speculate that a second FTZ cofactor that is spatially localized and recognizes homeodomain DNA binding sites interacts with this functionally redundant region of FTZ. We will initiate a yeast two hybrid screen to identify this second FTZ cofactor using the functionally redundant region as bait.
We initiated a study to find the homeodomain-dependent FTZ activity that we had proposed to operate during the cellular blastoderm stage (Hyduk and Percival-Smith 1996). In the course of this analysis we found that ectopically expressed FTZ was repressed by FTZ activity expressed in the endogenous FTZ stripes. We have also found a region of FTZ that may mediate this repression. We are presently determining what mediates this repression of ectopic FTZ expression.