

Environmental Stress Biology Group, Department of Biology, The University of Western Ontario
Who We Are-Research Projects-Publications-Positions Available-Contact
 Our main research focus is the study of the biosynthesis of suberin. Suberin is a plant cell wall modification that involves the deposition of both a poly(phenolic) and a poly(aliphatic) domain within the same tissue. As such, the deposition of suberin represents the coordinate regulation of both fatty acid metabolism (giving rise to suberin-specific aliphatic compounds) and phenylpropanoid metabolism (givin rise to suberin-specific phenolic compounds). Over the past decade, we have used chemical analyses to identify target metabolites unique to suberin and biochemical analyses to identify unique steps in their biosynthesis. We are now in a position to clone and characterize the genes that encode the enzymes for these unique biochemical steps. With these genes in hand, we will be able to ask questions about the regulation of suberization and study the mechanism(s) by which disparate pathways converge to make the unique material known as suberin.
Our main research focus is the study of the biosynthesis of suberin. Suberin is a plant cell wall modification that involves the deposition of both a poly(phenolic) and a poly(aliphatic) domain within the same tissue. As such, the deposition of suberin represents the coordinate regulation of both fatty acid metabolism (giving rise to suberin-specific aliphatic compounds) and phenylpropanoid metabolism (givin rise to suberin-specific phenolic compounds). Over the past decade, we have used chemical analyses to identify target metabolites unique to suberin and biochemical analyses to identify unique steps in their biosynthesis. We are now in a position to clone and characterize the genes that encode the enzymes for these unique biochemical steps. With these genes in hand, we will be able to ask questions about the regulation of suberization and study the mechanism(s) by which disparate pathways converge to make the unique material known as suberin.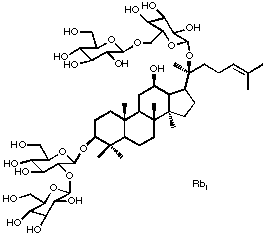
In addition, we are interested in the role(s) for which plants use secondary metabolites to interact with other organisms. Recently, we have focused on the role of ginsenosides (e.g., Rb1 at right) in the interaction between ginseng and one of its major pathogens, Pythium irregulare.
Our research group is located on the fourth floor of the North Campus Building, forming part of the Environmental Stress Biology Group. Other members of the ESBG include Dr. Norman Huner, Dr. Denis Maxwell and Dr. Charlie Trick.
Biology Faculty Biology Home Faculty of Science UWO Home
This site created and maintained, by Mark Bernards (bernards@uwo,ca). Last updated: March 2007

Who We Are-Research Projects-Publications-Positions Available-Contact-Back to top

"Da Boss"
(Dr. Mark A. Bernards)
Current Graduate and Undergraduate students in the lab...
PhD Students:
 |
Meg Haggitt | omega-Hydroxylases in Suberization |
MSc Students:
 |
Andreea Costea | Metabolism of Ginsenosides by Pythium irregulare |
| Stephanie Anderson | Phenolic Hydroxylases in Suberization | |
| Daniel Lee | Fatty Acid Elongation in Suberization |
Undergraduate Students:
| Daniel Klass | omega-Hydroxylases in Suberization |
Past Graduate Students
 |
Raymond Thomas, PhD 2006 | Soybean Root Suberin and Resistance to Phytophthora sojae |
 |
Suqin Shao, PhD 2006 | Soybean Seed Coat Cuticle |
 |
Wei-Li Yang, PhD 2006 | Metabolite Profiling of Suberizing Potato Tubers |
 |
Lina Yousef, MSc 2005 | Biotransformation of Ginsenosides by Microorganisms in vitro |
| Di Shao, MSc 2004 | Isolation of bioactive phytochemicals from Tripterygium wilfordii (Thunder God Vine) | |
| Clayton Crawley, MSc 2004 | Biochemical Characterization of a Prephenate Dehydratase-like Protein from Arabidopsis thaliana | |
| Rob Nicol, PhD 2003 | Allelopathic Chemicals in Ginseng (Panax quinquefolius) | |
| Fawzi Razem, PhD 2003 | H2O2 Production During Potato Suberin Formation | |
| Mirwais Qaderi, PhD 2002 | Germination Determinants in Scotch Thistle (Onopordum acanthium) | |
| Kristen Summerhurst, MSc 2002 | Immunocytochemical Localization of Suberization-Associated Peroxidases | |
| Xiaolong Yang, MSc 2000 | An Antimicrobial Compound from Impatiens balsamina | |
Past Undergraduate Students
Beatrix Czikkel, NSERC Summer Student (2004) Biochemical characterization of a plasma membrane NADPH-dependent oxidase
Wahid Pabani, NSERC Summer Student (2004) Novel hydroxycinnamic acid conjugate hydroxylase
Marie Davey, BSc Honors Project (2004) Clarifying phenylpropanoid metabolism: Sinapaldehyde and coniferaldehyde dehydrogenase activity during wound-induced suberization of potato tubers.
Rupa Murthi, BSc Honors Project (2004) Biochemical characterization of a plasma membrane NADPH-dependent oxidase
Mazen Al-Beldawi, BSc Honors Project (2003) Alkalinization of Tripterygium wilfordii Hook F. and its effect on pharmacological and toxcological activity
Ryan Dunne, BSc Honors Project (2002) Bio-transformation of ginsenosides by ginseng pathogens
Lina Yousef, BSc Honors Project (2002) Validation of colony diameter as a measure of fungal growth
Mike Gardner, BSc Honors Project (2001) Role of epoxides in the bioactivity of triterpenoids from Triptergium wilfordii
Jefferey Cowger, BSc Honors Project (2000) Bioactive compounds from Senecio scadens

Who We Are-Research Projects-Publications-Positions Available-Contact-Back to top
Suberin is a cell wall-associated biopolymer found in specific cell types, such as root epidermis, root endodermis (including the Casparian band), bundle sheath cells and the periderm (cork) of woody species and underground organs (e.g., tubers). Suberin is uniqe and distinct from other non-carbohydrate cell wall bio polymers such as lignin and cutin for several reasons: (1) it is an assembly of two distinct polymeric domains, one poly(phenolic) and the other poly(aliphatic), (2) each domain has a unique chemical composition, and (3) suberin is only found in specialized cells, as noted above. Suberin functions as a physical barrier, preventing water loss from the tissues it surrounds as well as providing protection against pathogens. It also forms a significant component of the wound healing process in plants; indeed the main model system to study suberin and the suberization process is the potato tuber. Upon wounding, tubers undergo a massive re-arrangement of metabolism, chanelling significant metabolic energy into the formation of the phenolic and aliphatic monomeric precursors of suberin via two disparate metabolic pathways.
Our research efforts have focussed on identifying unique metabolic steps associated with the biosynthesis of both phenolic and aliphatic suberin monomers, as well as aspects of the macromolecular assembly of the poly(phenolic) domain. Recent metabolite profiling experiments have been used to help delineate unique steps in suberin monomer biosynthesis. We now have identified a number of unique/critical metabolic steps in suberin monomer biosynthesis, and are working toward the cloning of the genes encoding the enzymes involved. With these clones in hand we will be able to more precisely study the coordinate regulation of critical genes in suberin biosynthsis.
Ecological Roles of Ginsenosides
Ginsenosides are triterpenoid saponins of the ginseng plant. Like other saponins, they are fungitoxic (although only slightly) and as such play a role in the interaction between ginseng plants and soil-borne microorganisms. One particularly aggressive pathogen of ginseng is the oomycete Pythium irregulare. This organism is able to metabolize common protopanaxadiol ginsenosides (e.g., Rb1, Rb2, Rc, Rd) via extracellular glycosidase(s) into a common product, ginsenoside F2. This compound may act as either a host recognition factor or a growth stimulant for P. irregulare, allowing it to find susceptible ginseng roots. Our current research focus is aimed at purifying the P. irregulare glycosidase responsible for ginsenoside biotransformation in an effort to better understand this economically important pathosystem.

Who We Are-Research Projects-Publications-Positions Available-Contact-Back to top
Book Chapters:
2. Bernards, M.A. and Båstrup-Spohr, L. Phenylpropanoid Metabolism Induced by Wounding and Herbivory. In: Induced Plant Resistance to Herbivory (Schaller, A., Ed). Springer Dordrecht The Netherlands. pp 189-211. 2008.
1. Bernards, M.A., Yousef, L.F. and Nicol, R.W. The allelopathic potential of ginsenosides. In: Allelochemicals: Biological Control of Plant Pathogens and Diseases (Inderjit and Mukerji, K.G., Eds). Springer Dordrecht The Netherlands. pp 157-175. 2006.
Peer-Reviewed Articles:
1980's 1990's 2000 2001 2002 2003 2004 2005 2006 2007
2008
2008
33. Qutob, D., Ma, F., Peterson, C.A., Bernards. M.A. and Gijzen, M.R. (2008) Structural and Permeability Properties of the Soybean Seed Coat. Canadian Journal of Botany (in press).
32. Shao, D., Dunlop, W.D., Lui, E.M.K. and Bernards, M.A. (2008) Immunostimulatory and Anti-inflammatory Polysaccharides from Tripterygium wilfordii: Comparison with Organic Extracts. Pharmaceutical Biology 46:8-15.
31. Cho, M.-H., Corea, O.R.A., Yang, H., Bedgar, D.L., Laskar, D.D., Anterola, A.M., Moog-Anterola, F.A., Hood, R.L., Kohalmi, S.E., Bernards, M.A., Kang, C., Davin, L.B. and Lewis, N.G. (2007) Phenylalanine Biosynthesis in Arabidopsis thaliana: Identification and Characterization of Arogenate Dehydratases. Journal of Biological Chemistry 282:30827-30835.
30. Yang, W. and Bernards, M.A. (2007) Metabolomic Analysis of Wound-Induced Suberization in Potato (Solanum tuberosum L.) tubers. Metabolomics 3:147-159.
29. Thomas, R., Fang, A., Ranathunge, K., Peterson, C.A. and Bernards, M.A. (2007) Soybean (Glycine max L.) Root Suberin I: Anatomical Distribution, Chemical Composition and Relationship to Phytophthora sojae Tolerance. Plant Physiology 144:299-311.
28. Nasir, M.I., Bernards, M.A. and Charpentier, P.A. (2007) Acetylation of Soybean Lecithin for Application In Supercritical Carbon Dioxide. J. Agric. Food Chem. 55:1961-1969.
27. Shao, S., Meyer, C.J., Ma, F. Peterson, C.A., and Bernards, M.A. (2007) The Outermost Cuticle of Soybean Seeds: Chemical Composition and Function During Imbibition. Journal of Experimental Botany 58:1071-1082.
26. Yang, W. and Bernards, M.A. (2006) Wound Induced Metabolism of Fatty Acids in Potato (Solanum tuberosum) Tubers. Plant Signaling and Behavior 1(2):59-66, 2006.
25. Wood, J.A., Bernards, M.A., Wan, W. and Charpentier, P.A. (2006) Extraction of Ginsenosides from North American Ginseng using Modified Supercritical Carbon Dioxide. Journal of Super Critical Fluids 39:40-47.
24. Qaderi, M.M., Cavers, P.B., Hamill, A.S., Downs, M.P. and Bernards. M.A. (2006) Maturation temperature regulates germinability and chemical constituents of Scotch thistle (Onopordum acanthium) cypselas. Can. J. Botany 84:28-38.
23. Yousef, L.F. and Bernards, M.A. (2006) In vitro metabolism of ginsenosides by the ginseng root pathogen Pythium irregulare. Phytochemistry 67:1740-1749.
22. Christov A., Korol, R.M., Dai, E., Liu, L., Guan, H., Bernards, M.A., Cavers, P.B., Susko, D. and Lucas, A. (2005) In vivo optical analysis of quantitative changes in collagen and elastin during arterial remodelling. Photochemistry and Photobiology 81:457-466.
21. Bernards, M.A., Summerhurst, D.K., and Razem, F.A. (2004) Oxidases, peroxidases and hydrogen peroxide: The suberin connection. Phytochemistry Reviews 3:113-126.
20. Qaderi, M.M., Cavers, P.B., and Bernards, M.A. (2003) Isolation and structural characterization of a water-soluble germination inhibitor from Scotch thistle (Onopordum acanthium) cypselas. Journal of Chemical Ecology 29:2425-2438.
19. Nicol, R.W., Yousef, L., Traquair, J.A. and Bernards, M.A. (2003) Ginsenosides stimulate the growth of soilborne pathogens of American ginseng. Phytochemistry 64(1):257-264.
18. Qaderi, M.M., Cavers, P.B. and Bernards, M.A. (2003) Pre- and post-dispersal factors regulate germination patterns and structural characteristics of Scotch thistle (Onopordum acanthium) cypselas. New Phytologist 159:263-278.
17. Razem, F.A. and Bernards, M.A. (2003) Reactive oxygen species production in association with suberization: Evidence for an NADPH-dependent oxidase. Journal of Experimental Botany 54:935-941.
16. Qaderi, M.M., Cavers, P.B. and Bernards, M.A. (2002) Seedbank Dynamics of Onopordum acanthium: Emergence patterns and chemical attributes. Journal of Ecology, 90, 672-683.
15. Nicol, R.W., Traquair, J.A. and Bernards, M.A. (2002) Ginsenosides as host resistance factors in American ginseng (Panax quinqufolius L.). Canadian Journal of Botany 80, 557-562.
14. Bernards, M.A. (2002) Demystifying suberin. Canadian Journal of Botany 80, 227-240.
13. Razem, F. A. and Bernards, M.A. (2002) Hydrogen peroxide is required for poly(phenolic) domain formation during wound-induced suberization. Journal of Agriculture and Food Chemistry 50, 1009-1015.
12. Yang, X., Summerhurst, D.K., Koval, S.F., Ficker, C., Smith, M.L. and Bernards, M.A. (2001) Isolation of an anti-microbial compound from Impatiens balsamina L. using bioactivity-guided fractionation. Phytotherapy Research 15, 676-680.
11. Bernards, M.A. and Razem, F.A. (2001) The poly(phenolic) domain of potato suberin: A non-lignin plant cell wall bio-polymer. Phytochemistry 57, 1115-1122.
10. Bernards, M.A., Susag, L.M., Bedgar, D.L., Anterola, A.M. and Lewis, N.G. (2000) Induced phenylpropanoid metabolism during suberization and lignification: A comparative analysis. Journal of Plant Physiology 157, 601-607.
9. Bernards, M.A., Fleming, W.D., Llewellyn, D.B., Priefer, R. Yang, X., Sabatino, A. and Plourde, G.L. (1999) Biochemical characterization of the suberin-associated anionic peroxidase of potato (Solanum tuberosum L.). Plant Physiology 121, 135-145.
8. Bernards, M.A. and Lewis, N.G. (1998) The Macromolecular Aromatic Domain in Suberized Tissue: A Changing Paradigm. Phytochemistry 47, 915-933.
7. Bernards, M.A., Lopez, M.L., Jajicek, J. and Lewis, N.G. (1995) Hydroxycinnamic acid polymers constitute the polyaromatic domain of suberin. Journal of Biological Chemistry 270, 7382-7386.
6. Nose, M., Bernards, M.A., Furlan, M., Zajicek, J., Eberhardt, T.L., and Lewis, N.G. (1995) Evidence for sequential biosynthetic steps during lignin polymerization. Phytochemistry 39, 71-79.
5. Eberhardt, T.L., Bernards, M.A., He, L., Davin, L.B., Wooten, J.B. and Lewis, N.G. (1993) Lignification in cell suspension cultures of Pinus taeda: In situ characterization of a gymnosperm lignin. Jounral of Biological Chemistry 268, 21088-21096.
4. Bernards, M.A. and Lewis, N.G. (1992) Alkyl ferulates in wound healing potato tubers. Phytochemistry 31, 3409-3412.
3. Bernards, M.A. and Ellis, B.E. (1991) Phenylalanine ammonia-lyase from tomato cell cultures inoculated with Verticillium albo-atrum. Plant Physiology 97, 1494-1500.
2. Bernards, M.A., Strack, D., Wray, V. and Ellis, B.E. (1991) Caffeoyl glucosides in fungal challenged tomato suspension cultures. Phytochemistry 30, 497-499.
1. Bernards, M.A. and Ellis, B.E. (1989) Phenylpropanoid metabolism in tomato cell cultures co-cultivated with Verticillium albo-atrum. Journal of Plant Physiology 135, 21-26.

Who We Are-Research Projects-Publications-Positions Available-Contact-Back to top
No positions are availiable at this time.