| HOME | GROUP MEMBERS | RESEARCH INTERESTS | PUBLICATIONS | CONTACT |
Solid State X-ray Structure of a Self-complementary AADD Hydrogen-Bonded Double Helix |
Research Interests |
|
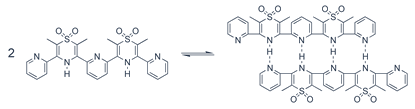
The primary focus of our group's research is the design, synthesis and characterization of new molecules that associate with each other in specific ways using multiple weak, non-covalent interactions. The use of these types of associations can lead to molecules and materials with unique properties that cannot be duplicated using covalent chemistry approaches alone. Some past and current projects are described below. New Hydrogen Bonding Subunits for Hydrogen Bonded Materials/Supramolecular Polymers - "Smart" Materials We have recently described the synthesis of an oligomer containing an ADADA (hydrogen bond Acceptor/Donor) hydrogen bonding array pictured below.
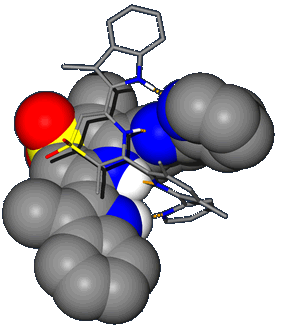
The complex formed by the hydrogen bonding between two strands has been drawn in a linear fashion for clarity. In reality, it requires that the two strands coil about one another to form a double helix lending a topological element to the formation of the complex that is not observed in most contemporary hydrogen bonded arrays. We have developed this chemistry to the stage where we can attach more advanced versions of these molecules to covalent scaffolds and generate hydrogen bonded materials and polymers that possess interesting reversible properties and are responsive to their environment (e.g. solvent or temperature changes). Recent designs incorporate light responsive components to add a further potential switching mechanism to the materials. Synthesis of Interlocked Molecules - Rudimentary Molecular Machines We have developed a new method of templating interlocked (or mechanically bonded) molecular structures that are formed through the use of second-sphere coordination of organic hosts to metal complexes. The products of this templation, termed rotaxanes (looking like a ring trapped around a dumbbell-shaped axle) and catenanes (looking like two successive links in a chain), are the precursors to rudimentary molecular machines such as molecular shuttles. An example shuttle synthesized in some of our recent research is depicted below.
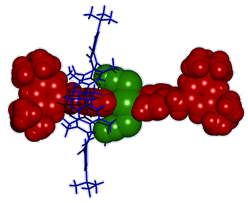
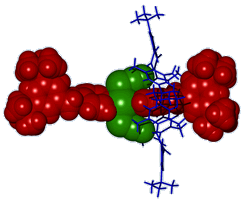
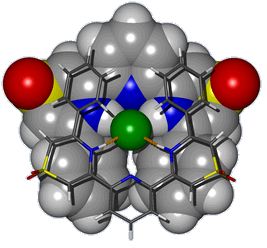
Hosts for Anions - Anion Recognition The selective complexation of anions by neutral host molecules is an active area of research in contemporary supramolecular chemistry due to their ubiquity in both natural and synthetic chemical systems. An offshoot of our interest in thiazine-1,1-dioxides as hydrogen bond donors is their incorporation as subunits in anion receptors. Some of these hosts display markedly different anion recognition properties to their analogous sulfonamide comparators. The simple incorporation of these thiazine dioxide subunits into oligomeric structures raises the intriguing possibility of creating anion responsive foldamers - molecules/materials that fold around anionic guests upon recognition. A chloride anion encapsulated in one of our prototypical receptors in the solid state is pictured below.
|