RNA INTERFERENCE
Induction of RNA interference for immunomodulation
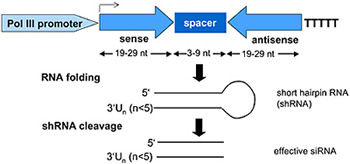
My laboratory is the first in the world to utilize short interfering RNA (siRNA) to silence immune associated genes in immune cells (PCT CA03/00867). This new technology allows us to potently and specifically silence gene expression across multiple cell generations. By blocking immune activating genes, we can generate large numbers of Tol-DC in vitro, which can be used clinically for treatment of autoimmune diseases and transplant rejection. (J Immunol. 2003, 171:691). Recently, we have developed 3 methods of inducing RNA interference using siRNA-expression cassettes (SEC), SEC-containing vectors, and plasmids encoding hairpin siRNA. We have successfully silenced MHC II, CD40, CD80, CD86, IL-12 and the transcription factor RelB in DC, 50 other immune-associated genes using siRNA.

siRNA-expressing vector
Transfecting Dendritic Cells with siRNA or siRNA-expressing vector
Treatment of autoimmune diseases by “tolerogenic vaccination” using siRNA modified Dendritic Cells
Using the murine model of rheumatoid arthritis, collagen II-induced arthritis (CIA), we demonstrated that administration of siRNA-IL12 treated DC, pulsed with CII, were sufficient to block progression of disease as observed by decreased pathological score, inhibited inflammatory infiltrate as seen by immunohistochemistry, and suppressed T cell and B cell responses as witnessed by dampened recall and antibody responses. Similar strategy has been also applied on EAE model by silencing TNF-alpha, and RelB genes. These studies suggested a potent tolerogenic vaccine that would be useful for treatment of autoimmune diseases.
Reinstallation of anti-cancer immunity by breaking tumor-derived immuno-suppression
Tumor-derived immune suppression in the main of cancer evasion from immune privilege, and is a major hurdle for the cancer immune/gene therapy. We developed a novel strategy to disrupt tumor-derived immune suppression by silencing tumor-originated tolerogenic molecule, indoleamine 2,3-dioxygenase(IDO), using small interfering RNA (siRNA). Murine melanoma cell line B16 expressed IDO, which can be efficiently silenced by siRNA specific to IDO (IDO-siRNA). IDO expressed in B16 induced T cell apoptosis and suppressed T cell responses in vitro and in vivo. Transduction of IDO-siRNA into B16 prevented calabolization of tryptophan, the most critical component for T cell survival, as well as inhibited T cell apoptosis. Silencing IDO in B16 in vitro inhibited tumor growth and disabled tumor formation in vivo after inoculation in syngeneic and allogeneic recipients. In addition, Treatment with IDO-siRNA in B16-bearing mice successfully postponed tumor formation time and significantly decreased tumor size. Furthermore, silencing IDO resulted in recovery of T cells responses and enhancement of tumor-specific killing. Taken together, silencing IDO may break tumor-derived immune suppression and RNA interference could be an alternative potential for caner therapy through reinstalling anti-cancer immunity.