|
Specific Research Projects |
|
Project I:
Pyroptosis and immunity |
|
Infectious disease is one of the major cause of illness and the
second leading cause of death world-wide. Understanding the host¡¯s
responses to microbes is fundamental to prevent and treat infectious
diseases. Many disease-causing microbes
release factors which kill macrophages to inhibit immune responses.
We found that anthrax lethal toxin and components
from influenza A virus cause rapid
macrophage cell death known as ¡°pyroptosis¡± which
is induced by defective mitochondria. To
date, the role and mechanism of pyroptosis are largely
unknown. This research project examines
the signaling/molecular mechanism of pyroptosis in macrophages and
its role in anthrax and influenza A virus infections.
Information obtained through this
research will provide
new therapeutic strategies in harnessing pyroptosis during
bacterial and viral infections. |
|
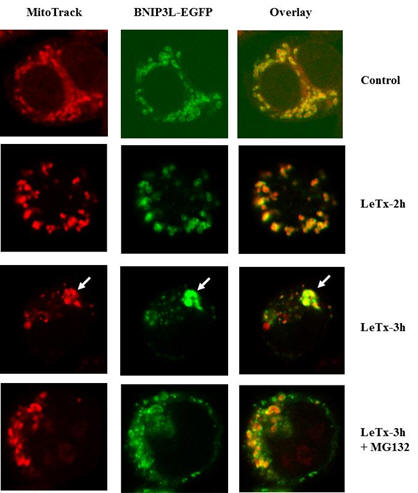
¡¡
|
LeTx rapidly induces
pyroptosis through mitochondrial
condensation and degradation.
Mitochondrial localization of GFP-Bnip3L were visualized using
confocal microscope in RAW264.7 cells after treating LeTx for 2 and
3 h in the presence or absence of proteasome inhibitor MG132 (20
µM). LeTx induced condensation or aggregation of Bnip3L (green) in
mitochondrial membrane as early as 2 h post-LeTx treatment.
Mitochondrial fusion or aggregation of fragmented mitochondria
(arrow) was also readily detected in LeTx-treated cells before
cytolysis. Mitochondrial potential (red) was greatly reduced after 3
h LeTx treatment |
|
Project II:
Epigenetic immune regulation by histone deacetylase 8 in macrophages |
|
Epigenetics is a cellular mechanism
that inheritably regulates gene expression without altering genomic
DNA sequences during development and cell differentiation. Recent
intense studies have focused on its role in development and cancers;
however, much less information is available on its role in immune
responses. Macrophages are key immune cells residing in all tissues,
and play a key role in directly killing microbes and orchestrating
subsequent immune responses. We found that a protein known as
histone deacetylase (HDAC) 8 plays a key role in modulating
macrophages in releasing inflammatory mediators known as cytokines.
This study examines the mechanism of HDAC8 in regulating macrophage
function and its role in bacterial infections. This research
will provide fundamental and detailed insights how our immune cells
interact with microbes through HDAC8, and the information obtained
through this research will provide new strategies for treating
various infectious and inflammatory diseases. |
|
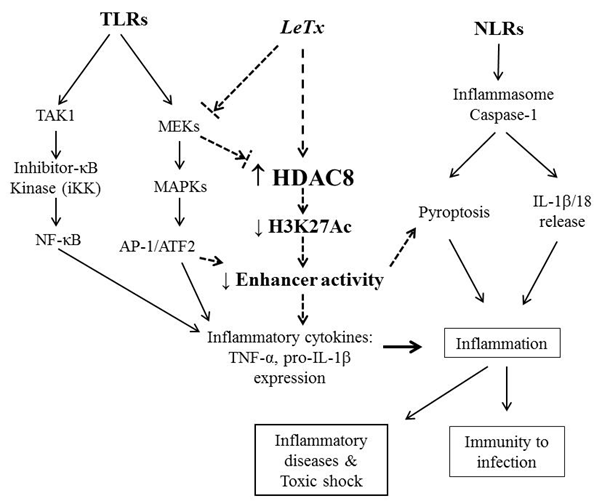 |
A proposed mechanism of HDAC8 in immune suppression
induced by anthrax lethal toxin (LeTx) and other virulence factors.
Activation of PRRs by microbial components induce
innate immune responses through activating various signaling modules
including MAPKs, NF-¥êB and the inflammasome. However, various
microbial virulence factors (bold italics) inhibit immune responses
through targeting these signaling molecules. LeTx inhibits both TLR
and NLR signaling events through preventing MAPKs and pyroptosis,
respectively. HDAC8 plays a key role in immune suppression induced
by LeTx, through targeting H3K27Ac and preventing enhancer
activities.
¡¡ |
|
Project III: Investigating
the mechanisms of immunomodulatory effects of probiotics. |
|
Commensal microbes live inside the
host in symbiosis. Probiotics are live commensals that have been
used for centuries to replenish normal or beneficial gut and
urogenital microflora. This research
project is to understand how the host
maintains immunological symbiosis with commensals and what
immunological effects probiotics have on the host. We
found that the probiotic strain of
Lactobacillus rhamnosus GR1 (GR1) and its secreted products have
immunomodulatory effects on macrophages, dendritic cells, intestinal
lamina propria cells and placental trophoblasts. GR1 as well as
other strains of Lactobacillus rhamnosus preferentially and potently
produce the immunomodulatory cytokine granulocyte colony-stimulating
factor (G-CSF) in these cells, which may play important roles in
maintaining immunological homeostasis of intestine and placenta.
This research proposal is to further study the cellular and
signaling mechanisms by which GR1, GR1-secreted factor(s) and G-CSF
in modulating macrophage differentiation, activation and survival.
Information obtained through this study will provide detailed
information on how lactobacilli modulate macrophage activation and
how G-CSF highly produced by lactobacillus-exposed macrophages
affects myeloid cell differentiation, cell
survival and gut homeostasis. |
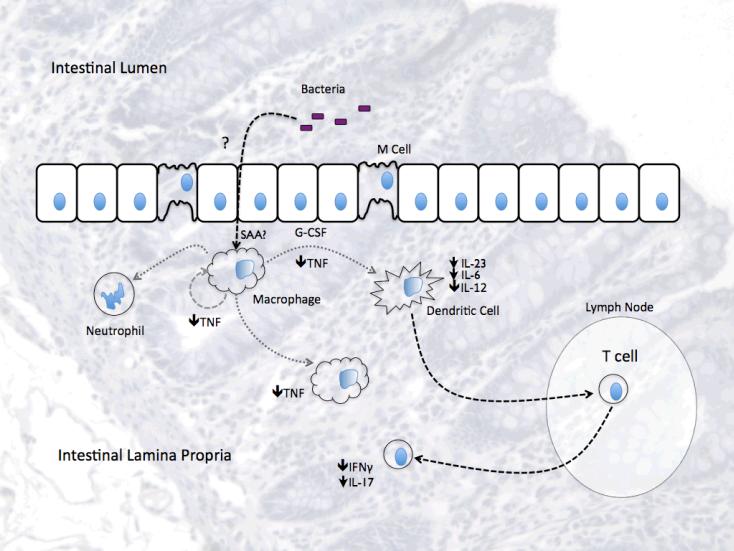
Martins A, Han J, Kim SO.
IUBMB Life. 2010 |
Model of the role of G-CSF in the
intestine. G-CSF may exert immunomodulatory effects in the intestine
through multiple mechanisms. Intestinal G-CSF may be produced by
macrophages or subepithelial myofibroblasts, induced or modulated by
luminal bacteria or endogenous factors such as serum amyloid A (SAA).
G-CSF can then act locally to strengthen the barrier protecting
epithelial cells from injury-induced apoptosis and stimulating
neutrophil effector function to aid in the clearance of translocated
bacteria. At the same time, G-CSF may have immunoregulatory effects
on macrophages, dendritic cells, as well as Th1 and Th17 responses.
¡¡ |


