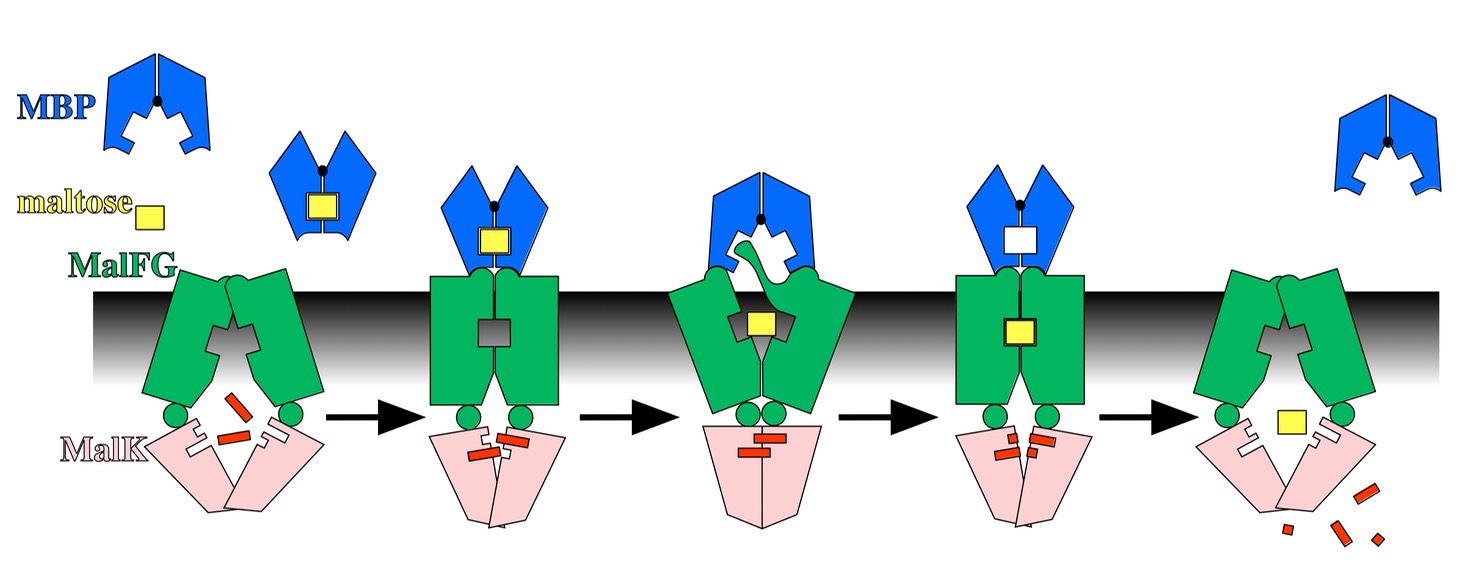
ABC Transporters are found in all living systems and have a common architecture: membrane integral subunits (green) that have a substrate binding site, with two ATP Binding Cassettes (pink) that power the system by binding and hydrolyzing ATP.
Many ABC transporters are exporters, and are often involved in movement of toxic molecules out of the cell. Most notorious are the multidrug transporters, which enable tumour cells to survive chemotherapy, and bacteria to evade antibiotics. There are also ABC import systems that are critical for nutrient acquisition and survival of bacteria in diverse environments.
With numerous crystal structures and a wealth of biochemical and genetic data, the Maltose Transporter from E. coli is providing us with a molecular-level understanding of how the individual subunits undergo concerted conformational changes, and how movement of maltose across the membrane is coupled to hydrolysis of ATP.