|
General overview:
We aim at identifying the role played by protein
glycosylation in the virulence of two genetically related
gastro-intestinal human pathogens that cause very different and specific
pathologies: Campylobacter jejuni and Helicobacter pylori. We have made
great progress in the elucidation of the N- and/or O-linked protein
glycosylation pathways in these bacteria, and in the determination of their
role in pathogenesis. We are now investigating the role of glycosylation on
the function of select glycoproteins.
Other significant research topics in the laboratory include investigating the
biosynthesis and role of C. jejuni
capsular components, the role of amoeba in survival
of C. jejuni
in the environment and host transmission, and the function of novel secreted proteins in the virulence of H. pylori.
|

|
|
Figure 1: C. jejuni.
|
|
1/ Background:
Campylobacter jejuni is the leading cause of endemic and traveler's bacterial gastroenteritis.
It has also recently been linked to the development of intestinal cancers.
The endemic character of C. jejuni
infections in developing countries, together with the high incidence of C.
jejuni associated traveler's disease cases,
and the emergence of antibiotic resistant clinical isolates of C. jejuni emphasize the need for the discovery of new
potential therapeutic targets. However, this will require a better
understanding of the virulence factors of this organism and of their specific
roles during host infection and colonization.
Helicobacter pylori was
only discovered in the early eighties by Warren and Marshall, who received
the 2005 Physiology and Medicine Nobel Prize. Since then, H. pylori has
been shown to be responsible for gastric ulcers and
cancers. It is present chronically in 70-90% of the population in
developing countries but its mode of transmission and its potential initial
reservoir are not known. This organism is very well adapted to the hostile
environment of the human stomach in which it thrives. Although numerous
virulence factors have been identified, the pathogenesis of H. pylori
is still far from fully understood. Additional factors that control the high
stringency of host specificity and that could be critical for host
colonization have yet to be identified and characterized.
2/ Research focus:
2.1/ Protein glycosylation in Campylobacter jejuni and Helicobacter pylori:
A common hallmark of these bacteria is the production
of glycoproteins that contribute significantly to their virulence.
Using a combination of bacterial genetics, microbiology and enzymology, we have made great
progress in the elucidation of the biosynthetic pathways that generate the
sugars that are necessary for protein glycosylation in both bacteria.
We have characterized several key enzymes of these pathways at the
biochemical and/or structural level (Creuzenet et
al 2000; Creuzenet 2004; Obhi
et al 2005; Vijayakumar et al 2006; Ishiyama et al 2006; Demendi
and Creuzenet 2009) and produced evidence to
suggest the existence of multiple glycoproteins in H. pylori (Hopf et al 2011). We have
also produced knockout mutants of these enzymes in both bacteria, and
analyzed how the mutations affected the production and function of virulence
factors such as flagella, lipopolysaccharide and urease (Merkx-Jacques
et al 2004; Vijayakumar et al 2006; Hopf et al 2011). Some of the mutants were also tested in
animal models. Using similar techniques, we are now focusing on determining
the role of protein glycosylation on the function of select glycoproteins.
All our data indicate that the enzymes targeted are important for the
production of multiple virulence factors and represent good candidates for
the development of inhibitors with potential therapeutic value.
2.2/ Biosynthesis of modified
heptoses in Campylobacter jejuni and Yersinia pseudotuberculosis:
C. jejuni produces a
capsule that is also essential for its virulence. It comprises uniquely
modified heptoses that do not exist in Mammals. Consequently, the enzymes involved in their synthesis are potential new
targets for therapeutic intervention against Campylobacter species. At
onset of this research, the biosynthesis of these modified heptoses was not
understood. Using a combination of bacterial genetics, cellular biology and
microscopy techniques, we have identified 3 genes that are
responsible for the synthesis of these modified heptoses, and shown that each
of them contributes to the full virulence potential of C. jejuni strain NCTC
11168, both in vitro
and in an animal model (Wong et al 2015). We are in the process of
characterizing the encoded enzymes at the biochemical level, to allow
subsequent development of inhibitors. We have characterized the enzymes
involved in heptose modification in strain NCTC 11168 (McCallum et al
2013) and also fully characterized the heptose modification pathway of a highly virulent strain
of C. jejuni
that produces a slightly different heptose
derivative than strain NCTC 11168 and revealed unexpected enzymatic
activities which can be exploited for the development of inhibitors (McCallum
2011; McCallum 2012). A comparative structural and functional study of the
enzymes involved in both strains is currently under way to determine common
features that could be targeted for inhibitor development.
Similar modified heptoses are also found in the
lipopolysaccharide of another gastro-intestinal pathogen, Yersinia
pseudotuberculosis but their contribution to
the role of capsule or LPS as virulence factors and their biosynthetic
pathways were unknown. Using a combination of molecular genetics and mass
spectrometry analyses, we have identified the 2 genes
responsible for 6-deoxyheptose biosynthesis in Y. pseudotuberculosis
and shown that the 6-deoxyheptoses are important for resistance to components
of the host’s innate immune defenses both in vitro and in vivo.
(Ho et al 2008; Kondakova et al 2008). The enzymes
involved were fully characterized at the biochemical level (Butty et al 2009).
|
|
|
Figure 2:
H. pylori
|
|
2.3/ Role of novel secreted proteins in
the virulence of Helicobacter pylori:
We have identified novel secreted proteins of unknown function in H. pylori. We have demonstrated that
these proteins are produced and secreted in laboratory strains. With the help
of our collaborators Prof. C. Burucoa and Dr. H. Atanassov (Universite
de Poitiers, France),
we were able to show that our proteins of interest are produced by clinical
isolates of H. pylori and that patients with H.
pylori infection produce antibodies against these proteins. We are
currently investigating the function of these proteins using tissue culture
and in vivo models. We are also investigating the mechanism of folding and
secretion of these proteins as this mechanism could be targeted by
therapeutic inhibitors to prevent the secretion of our proteins of interest. We
have identified a novel chaperone involved in their folding prior to
secretion and performed an extensive biochemical and fucntional charactreization of this protein (Lester et al 2015).
2.4/
Role of amoeba in survival of Campylobacter
jejuni in the environment:
Transmission of C. jejuni to humans from contaminated
poultry is recognized as an important socio-economic and health problem. The
mode of transmission of C. jejuni from the environment to poultry is not well
understood. We recently determined that amoeba could play a role in the
process. Contrary to the accepted dogma, we showed that intra-amoeba survival
of C. jejuni
is very limited. Instead, we showed that C.
jejuni benefits from amoeba-mediated depletion
of dissolved oxygen from the environment to thrive extracellularly
in aerobic conditions (Bui et al
2012a). This may play an important role in the chain of contamination of new
poultry flocks in broiler houses. We also investigated whether environmental
exposure to stress affected the subsequent interactions of C. jejuni
with amoeba, which in turn could affect dissemination (Bui et al 2012b). We
are currently investigating if protein glycosylation or capsular heptose modification have any effect on the interactions
of C. jejuni
with amoeba.
3/ What you
would get to do if you joined us as a Graduate student?
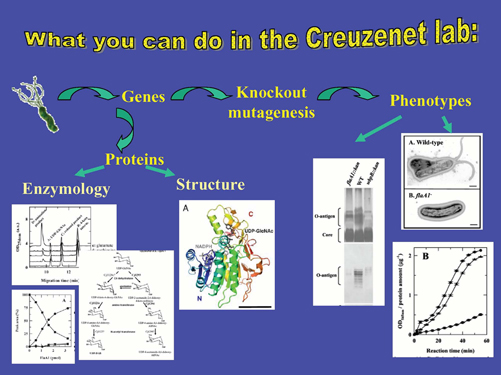
You would be in charge of a full project, covering genetic and functional
aspects. This would provide you with have ample opportunity to acquire
training in multiple disciplines, ranging from basic Microbiology to sugar
and protein Biochemistry, encompassing Molecular Biology and Cell Biology.
You would get exposed to state-of-the-art techniques such as confocal microscopy and capillary electrophoresis as well
as to traditional but ever so useful techniques such as HPLC/FPLC
purifications of enzymes and sugars, cloning, PCR,
electrophoresis, Western blotting, tissue culture etc…
To inquire about available positions, please
email your CV and transcripts to Dr Creuzenet at ccreuzen@uwo.ca.
[Back Home] [The Team] [Publications] [Relevant Links]
|



