Research Interests
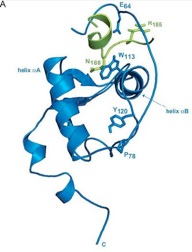
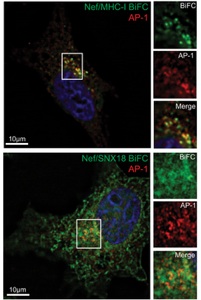
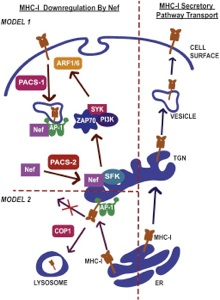
In addition to the virally encoded enzymes required for replication and assembly, HIV-1 expresses a collection of accessory proteins that lack intrinsic enzymatic activity but which are essential for disease pathogenesis by dysregulating host cell enzymatic activities to counterattack the host antiviral response and promote virus replication. In particular, the HIV-1 accessory protein Nef is required for the efficient onset of AIDS following HIV-1 infection. Nef modifies the host cellular environment in many ways, including alteration of T cell activation, modulation of apoptotic and autophagic pathways, as well as disrupts the intracellular trafficking of MHC-I and other cell surface molecules of helper T cells and macrophages. Our laboratory is interested in the various interactions between Nef and host cellular partners and how these interactions modulate membrane trafficking pathways to evade the immune system.