






Research Projects in the Mymryk Laboratory
General Overview
It is estimated that viruses are responsible for about 15% of human cancers. In this respect, viruses are a risk factor for cancer second only to tobacco. Human papillomavirus (HPV) infection is thought to account for about two-thirds of these cancers. In particular, HPV infection is responsible for nearly all cervical cancers and the majority of oropharyngeal cancer. Infection with HPV forces quiescent cells to enter the cell division cycle. This abnormal cell proliferation initiates progression to the cancer phenotype in combination with virally induced chromosomal instability, evasion of the host immune response and suppression of programmed cell death. E6 and E7 are the two key viral proteins that are required for these activities. E7 binds pRB, the product of the retinoblastoma tumor susceptibility locus, which is an important regulator of cell growth. This interaction is not sufficient for the effects of E7 on cell growth, indicating that E7 targets additional cellular proteins.
Human adenovirus differs from HPV in that it they are not known to be associated with human cancers. However, they can oncogenically transform cells in culture, providing an important model for cancer research. The adenoviral E1A and E1B oncogenes encode two key viral proteins required for transformation. Like E7, E1A binds pRB and many other cellular regulatory proteins.
We are interested in discovering new targets of the E1A and E7 oncoproteins and learning more about their effects on cellular functions. We use genetic and biochemical approaches to study their effect on cell growth. Our goal is to use these viral oncogenes as tools to identify and characterize cellular regulatory pathways that, when altered, contribute to cancer formation and its spread throughout the body. Surprisingly, the E1A proteins also possess anti-oncogenic properties and can suppress oncogenic transformation, tumorigenicity and metastasis. For this reason, studies of E1A function will help us identify events that can contribute to tumour formation as well as allow us to identify mechanisms that can inhibit the spread of cancer in an afflicted individual.
Molecular and Cellular Biology of Adenovirus E1A
The Early Region 1A (E1A) proteins of human adenovirus target multiple cellular regulatory proteins and these interactions form the molecular basis by which E1A alters cell growth and gene expression.
We undertake molecular genetic analyses of E1A function that identify new targets of E1A and the protein binding motifs mediating these interactions. We us E1A as a tool to identify critical regulators of cell growth and gene expression that are highly relevant to cancer. Oour studies will help us understand how these targets contribute to human cancer.
For more information on E1A, please have a look at the Adenovirus 5 E1A Page.
This work is funded by the CIHR.
Molecular and Cellular Biology of Human Papillomavirus E7
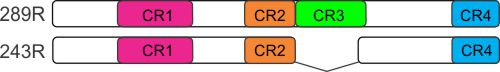
The HPV E7 proteins target multiple cellular regulatory proteins and these interactions alter cell growth and gene expression. We are undertaking a detailed struncture-function analysis of HPV16 E7 function. E7 is comprised of three regions that are conserved between the E7 protein of multiple HPV types. Our work focuses on a detailed structure function analysis of conserved region three (CR3), which binds zinc and stably folds to create a novel three-dimensional structure (see below) that confers interaction with multiple cellular regulatory proteins.
This work is funded by CIHR.

Studies of Non-conventional Nuclear Import Signals
Proper protein function often requires targeting to particular sub-cellular compartments via specific localization signals. Nuclear import of cellular and viral proteins is typically mediated by their physical interaction with soluble cytosolic receptor proteins via nuclear localization signals (NLS). We are interested in the identification of non-conventional nuclear import signals and understanding how they function. Many viral proteins, including adenovirus E1A, localize to the nucleus via poorly understood mechanisms, providing ideal systems to explore this area. As nuclear transport regulates, at least in part, an extraordinarily diverse range of cellular processes including cell cycle, signal transduction, gene expression, apoptosis and circadian rhythm, the identification of mechanisms regulating nuclear import is a valuable area of investigation. Much of this work is done in yeast, using Saccharomyces cerevisiae as a model system. We developed an improved transcription based assay to detect nuclear import, which we continue to use.
This work is currently funded by by NSERC.

Molecular and Cellular Biology of Human Cytomegalovirus (CMV)
This is an ongoing collaboration with Dr. Laura Hertel that is funded by the CIHR.
We are interested in CMV proteins that localize to the mitochondria and mechanisms to explain viral tropism for different cell types.

Translation Head & Neck Cancer Research
This is an ongoing collaboration with Dr. Anthony Nichols, a Clinician Scientist/Head & Neck Surgeon, and Dr. John Barrett in the Department of Otolaryngology.
Human papillomavirus (HPV) currently causes ~70% of orophryngeal tumours. The frequency of this virally induced cancer continues to increase dramatically and is considered to be an epidemic.
Understanding how the HPV virus causes oral cancers and how the presence of viral oncoproteins influences response to therapy will help improve diagnosis and treatment.
Link to the The Translational Head and Neck Cancer Program
Picture below: Drs. Barrett, Nichols and Mymryk growing beards for Manuary in 2012.

Funding Agencies



go back to main page














