Chemistry in Small Volumes: from mesoscopic clusters to biological cells

Droplets appear under different guises in many aspects of everyday life and technology. We observe them in naturally occurring atmospheric aerosols, in industrial and household sprays, as vesicles in nanofluidics and microfluidics, as emulsions and as precursors of phase-separation processes to mention but a few examples. Droplets are often charged due to the presence of simple ions and macroions (e.g. nucleic acids, proteins and other polyelectrolytes). As it has been demonstrated in recent electrospray-collision beam experiments [see experiments of G. Cooks at Purdue University and R. N. Zare at Stanford] droplets provide a distinct environment for chemical reactions where certain reactions become orders of magnitude faster than their bulk analogues. For this reason, chemistry in the small volume of the droplets may be the future ``beaker'' of chemistry. From another perspective, a cell and certain of its organelles share common features with a droplet. These features include confinement, crowding and shape fluctuations. Because of these commonalities, a droplet may be used as a model of a biological cell. Considering the broadness of the droplet world, discovering of the ion and macroion-droplet interactions is of central importance in atmospheric chemistry, biology, technology applications, analytical chemistry, microfluids and nanofluidics. We examine the following topics in droplet chemistry:
Atmospheric and Electrosprayed Aerosols
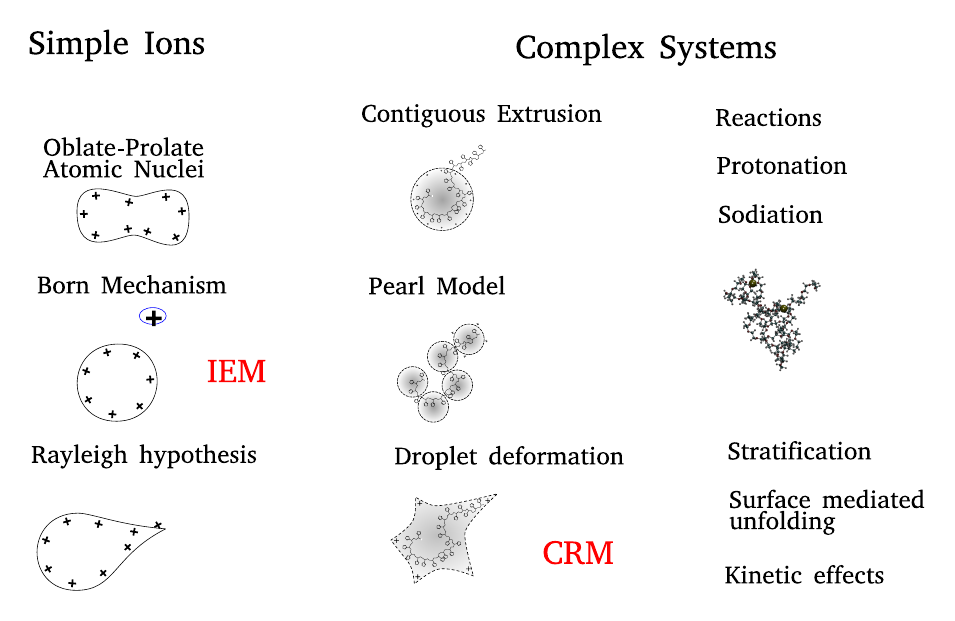
Classification of (macro-)ion-drop interactions
From Special Seminar at Lawrence Berkeley National Laboratory

The chemical and physical processes that occur in aerosols as well as
the presence of aerosol particles themselves play a critical role in
the climate, visibility in the atmosphere, quality of air, and
health. Because of the immediate effects of aerosols in everyday life
and their induced long term effects in climate and health, they have
attracted numerous studies over several decades.
Despite the many efforts to understand the
chemistry of aerosols they are still poorly understood in terms of the
reactions that take place in the aerosol substrate as well as the role
of nucleation and growth on the formation of the larger aerosol
particles. To add to the still limited knowledge of their behavior,
aerosols is one of the most uncertain factors in predicting future
climate change. The haze pollution in Beijing is
one of the most serious, if not the most serious case of detrimental
aerosol effect on human health, visibility conditions and weather.
Often aerosols are charged (positively or negatively) because of the presence of
simple ions. It has been established that aerosols
are also composed of biological material such as bacteria,
fungal spores and fragments, pollen, viruses,
biological crusts and lichens and others like plant or animal
fragments and detritus. The manner
that the biological material and in general organic material may affect nucleation processes in the
atmosphere has not been studied so far. Using molecular modeling
we examine interconconversion reactions of ions in mesoscopic clusters, which are
also called nano-drops, and the role
of biological material in the nucleation of particles and growth.
We have developed methods and software to compute the instantenous and average electric
field in nano-drops, shape fluctuations, surface tension and dielectric constant.
By using scaling and extrapolation we have found how certain physical properties
transition from the nano-drops to micro-drops.
V. Kwan and S. Consta ``Molecular characterization of the surface excess charge layer
in droplets'' accepted Journal of the American Society for Mass Spectrometry (2020).
V. Kwan and S. Consta ``Bridging electrostatic properties between nanoscopic and
microscopic highly charged droplets'' Chemical Physics Letters, 746, 137238 (2020).
V. Kwan, A. Malevanets, S. Consta ``Where do the ions reside in a highly charged droplet?''
Journal of Physical Chemistry A, 123(43): 9298--9310 (2019).
A. Malevanets, S. Consta ``Variation of droplet acidity during evaporation''
Journal of Chemical Physics 138 (18): 184312 (2013).
S. Consta and R. Kapral. ``Ionization reactions of ion complexes in mesoscopic
water clusters.'' Journal of Chemical Physics 111:10183-10191 (1999).
Star-shaped droplets
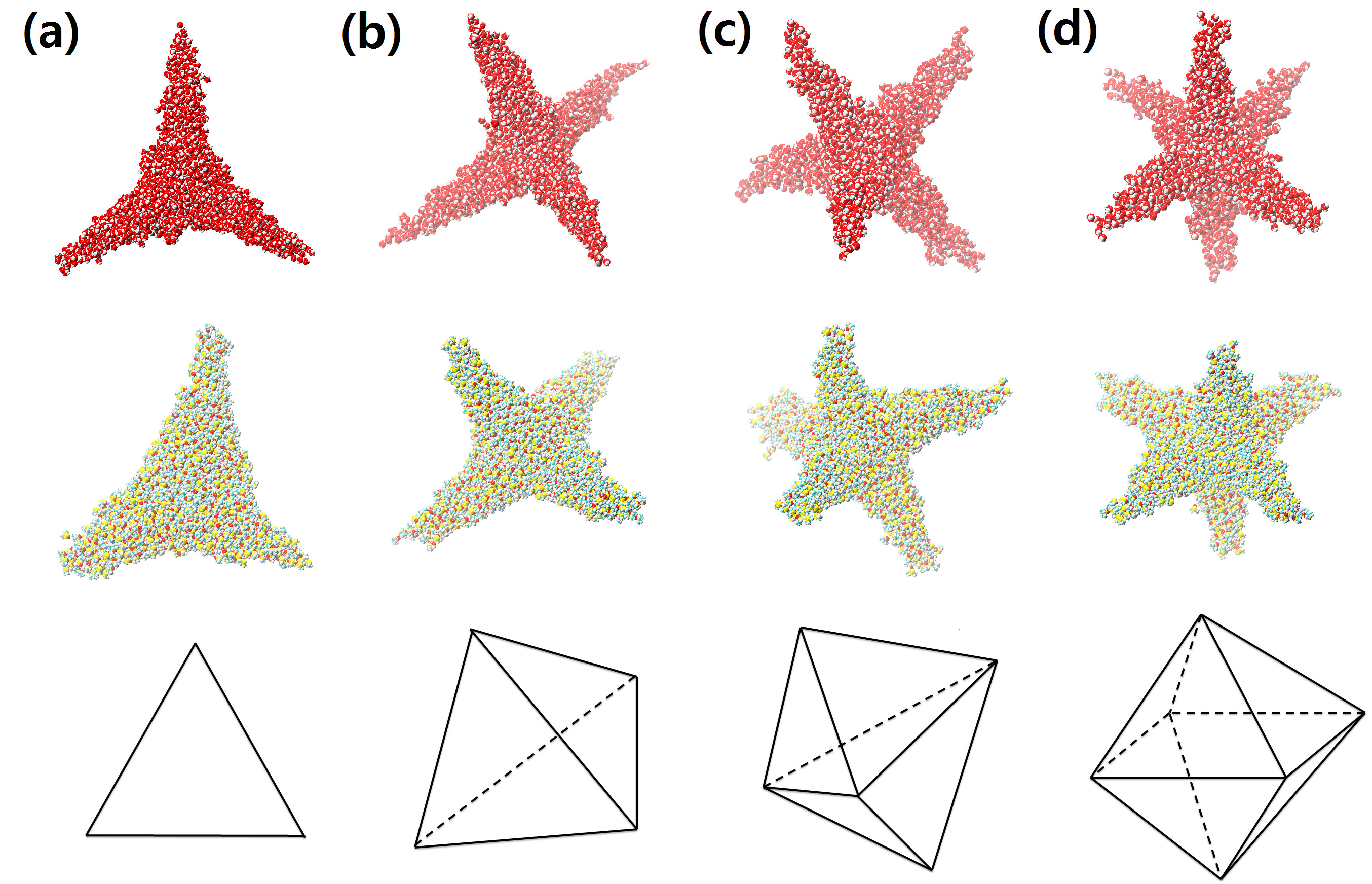
An important result of our research is the discovery that a droplet
comprising a charged macroion and solvent under certain conditions may
form ``star''-shapes with highly non-convex geometry.
These shapes have the same morphology as branched colloidal
nanocrystals. The fabrication of
mono-disperse nano-crystal building blocks of complex geometry is a
coveted goal in the assembly of functional nanostructures. Our
discovery may open a new direction in production of solid highly
non-convex colloids by adjusting the charge state of the central
macroion. We analyze distinct features in the structure of the
star-shaped nano-droplets using molecular and continuum modelling.
A. Malevanets, M. In Oh, M. Sharawy, S. Consta
``Landau-Ginzburg theory for the ``star''-shaped droplets''
Molecular Physics, 116.21-22: 2892-2900 (2018).
M. In Oh, A. Malevanets, M. Paliy, D. Frenkel,
S. Consta ``When droplets become stars: charged dielectric droplets beyond the
Rayleigh limit'' Soft Matter 13: 8781-8795 (2017).
M. Sharawy and S. Consta ``Characterization of ``star'' droplet morphologies induced by
charged macromolecules'' The Journal of Physical Chemistry A 120: 8871-8880 (2016).
S. Consta ``Manifestation of Rayleigh instability in droplets containing multiply
charged macroions'' Journal of Physical Chemistry B 114(16): 5263--5268 (2010).
Assembly of Stars
Dissociation rates and reaction pathways
The difference in the free energies between chemical species and the
rates of the relaxation towards the equilibrium are the two most
fundamental questions in chemistry. Analysis of protein and ligand
binding reactions, protein and macromolecular assemblies require
accurate and efficient methods for the reaction rate calculations.
The processes often occur on timescales that are yet inaccessible to
molecular dynamics methods. Molecular dynamics simulations in the
microsecond-millisecond range are still very much the exception while
the typical timescale of protein unfolding is in the order of milliseconds to microseconds.
Techniques for accurate calculations of free energies and reaction
rates are important for their practical and fundamental
aspects. A number of factors determine the accuracy of the
free energy calculations. Among the most important ones are the
sampling efficiency and the dominant rate of equilibration in the
system. We apply first principles in order to determine the reaction
pathways and compare the exact results with the available techniques.
S. Consta ``Detecting reaction pathways and computing reaction rates
in condensed phase'' Theoretical Chemistry Accounts 116 (1-3): 373--382 (2006).
S. Consta, K. Mainer and W. Novak ``Mechanisms of fragmentation processes of aqueous
clusters charged with ions'', Journal of Chemical Physics 119(19):10125 (2003).
S. Consta and R.~Kapral ``Dynamics of proton transfer in mesoscopic clusters.''
Journal of Chemical Physics, 104(12):4581--4590 (1996).
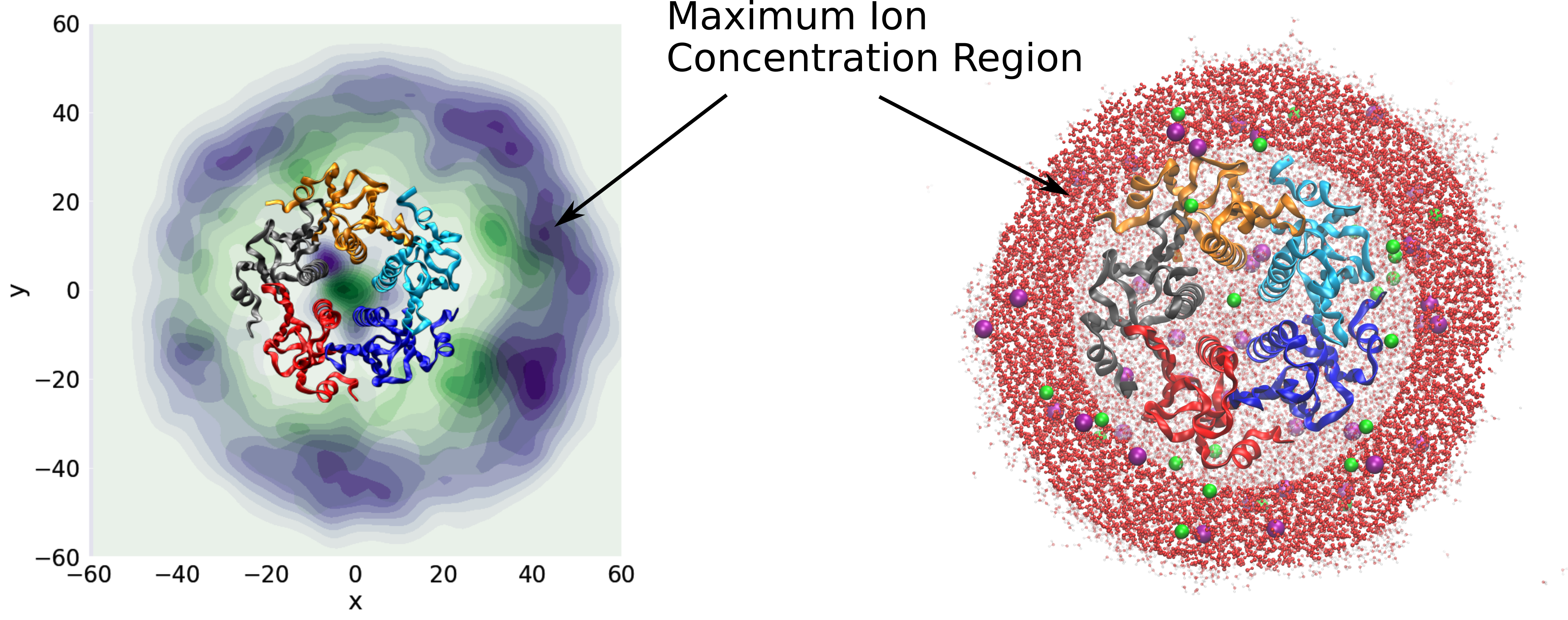
Macroion-ion interactions in droplet and bulk solution
The charge state of large macroions depends on the environment.
We investigate reactivity and ion distribution in charged droplets in the presence
of DNA, RNA, intrinsically disordered proteins and protein assemblies . We compare the results
of atomistic modelling with the solution of the non-linear Poisson-Boltzmann
equation. The solvers for the non-linear Poisson-Boltzmann equation have been
developed in the group.
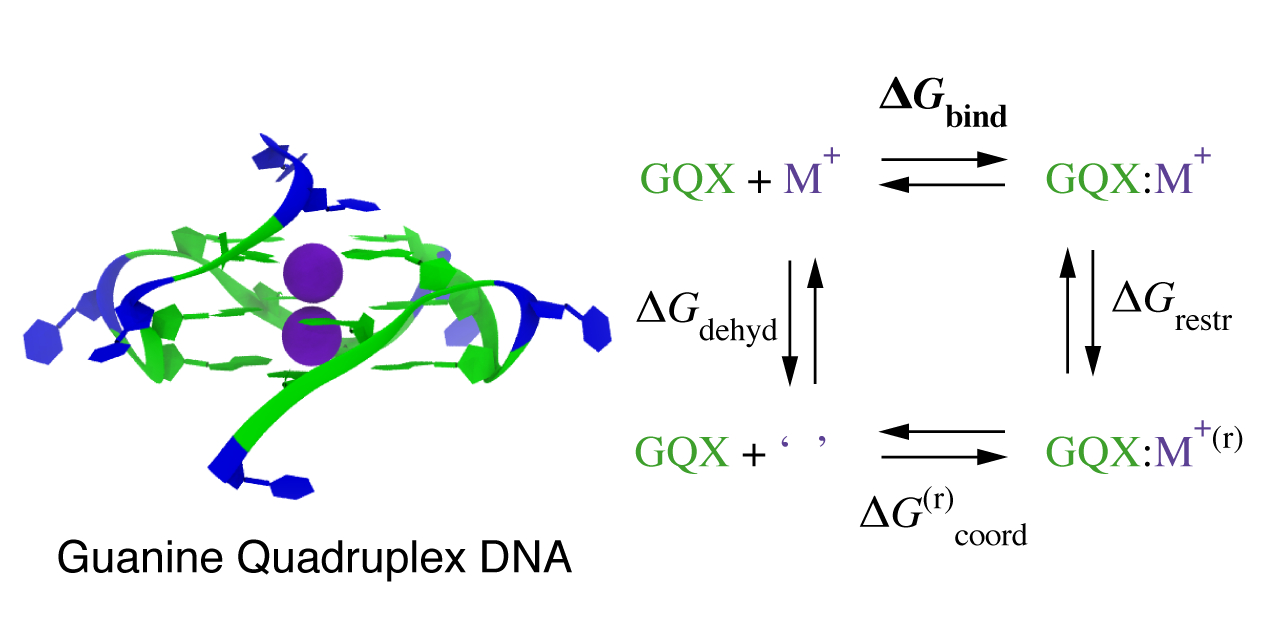
M. Sharawy, S. Consta ``Effect of the chemical environment of the DNA
guanine quadruplex on the free energy of binding of Na and K ions''
Journal of Chemical Physics, 149.22: 225102 (2018).
S. Consta, A. Malevanets, M. In Oh, M. Sharawy,
Molecular Simulation ``Role of a reaction coordinate in free energy calculations''
44:1033-1043 (2018).
S. Consta, M. In Oh, M. Sharawy, A.~Malevanets
``Macroion-solvent interactions in charged droplets'',
Invited {feature and cover article}
in the Journal of Physical Chemistry A, {\bf 122}(24), 5239-5250 (2018).
S. Consta, Myong In Oh, A. Malevanets ``New mechanisms of macroion-induced disintegration
of charged droplets'' Invited article in Chemical Physics Letters-Frontiers
{\bf 663}: 1--12 (2016).
M. Sharawy and S.~Consta ``How do non-Covalent Complexes
Dissociate in Droplets? A case study of the Desolvation of dsDNA from a Charged Aqueous Nanodrop''
Physical Chemistry Chemical Physics 17: 25550-25562 (2015).
S. Consta and A.~Malevanets ``Manifestations of charge induced instability
in droplets effected by charged macromolecules'' Physical Review Letters 109(14):
148301 (2012).
S. Consta and Jun Kyung Chung ``Charge-Induced Conformational Changes of
PEG-$(\rm{Na}^{+})_{n}$ in vacuum and aqueous nanodroplets'' Journal of Physical
Chemistry B 115(35): 10447--10455 (2011).
Protein-protein and protein-ligand interactions in dimeric complexes and larger assemblies
Protein complexes offer seemingly an inexhaustible collection of
reaction mechanisms for complex assembly. We have studied
the stability of a protein complex in
droplet and bulk solution. Rational drug design endeavours to determine new ligands based on the
knowledge of the target protein receptor. Binding a ligand to the
receptor induces a change in its respective protein biological
function. Precise calculation of the affinities or, alternatively,
free energies of protein-ligand complex formation is a challenging
problem as it necessitates exhaustive sampling ligand and protein
conformations. Finding a cost-effective method for proteome-wide
affinity determination is a critical step towards the discovery of new
drugs.
S. Consta, M. Sharawy, M. In Oh, A.~Malevanets
``Advances in modeling the stability of non-covalent complexes
in charged droplets with applications in ESI-MS experiments''
Perspective article in Analytical Chemistry 89: 8192--8202 (2017).
M. In Oh, S.~Consta What Factors Determine the Stability
of a Weak Protein-Protein Interaction in a Charged Aqueous Droplet?
Physical Chemistry Chemical Physics {\bf 19}: 31965--31981 (2017).
Myong In Oh and S.~Consta ``Stability of a Transient Protein Complex in a Charged Aqueous
Droplet with Variable pH'', Journal of Physical Chemistry Letters, 8: 80-85 (2017).