Welcome to the Consta Computational Chemistry Lab
Our research program seeks to push the boundaries of chemistry by advancing the fundamental understanding of chemical mechanisms under confinement. Droplets provide a particularly important and versatile example of confined environments. At these length scales, surface effects dominate, making interfacial chemistry a central focus of our work.
We investigate a broad range of phenomena in droplets, including:
-
Ion transport processes in droplets found in both natural and anthropogenic aerosols, as well as in ionization methods used in mass spectrometry
-
The supercooled state of water within droplets
-
Reaction acceleration in confined droplet environments
-
Conformational changes and charging mechanisms of macroions (e.g., proteins and nucleic acids) in droplets and their relationship to their behavior in bulk solution
-
The evolution of chemical reactivity and physical properties as a function of droplet size, from the micrometer scale down to nanometer dimensions
-
Usage of a droplet as a model for transport processes in a biological cell
To address these challenges, we have developed a suite of multiscale modeling approaches that integrate continuum descriptions, atomistic simulations, quantum ab initio methods, and numerical solutions of electrostatic equations.
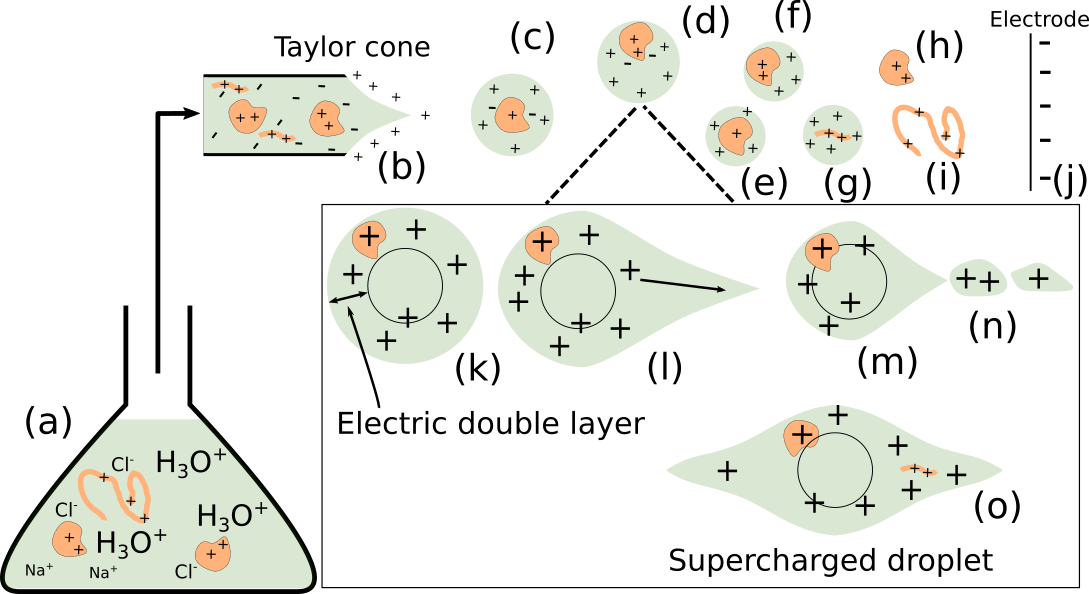
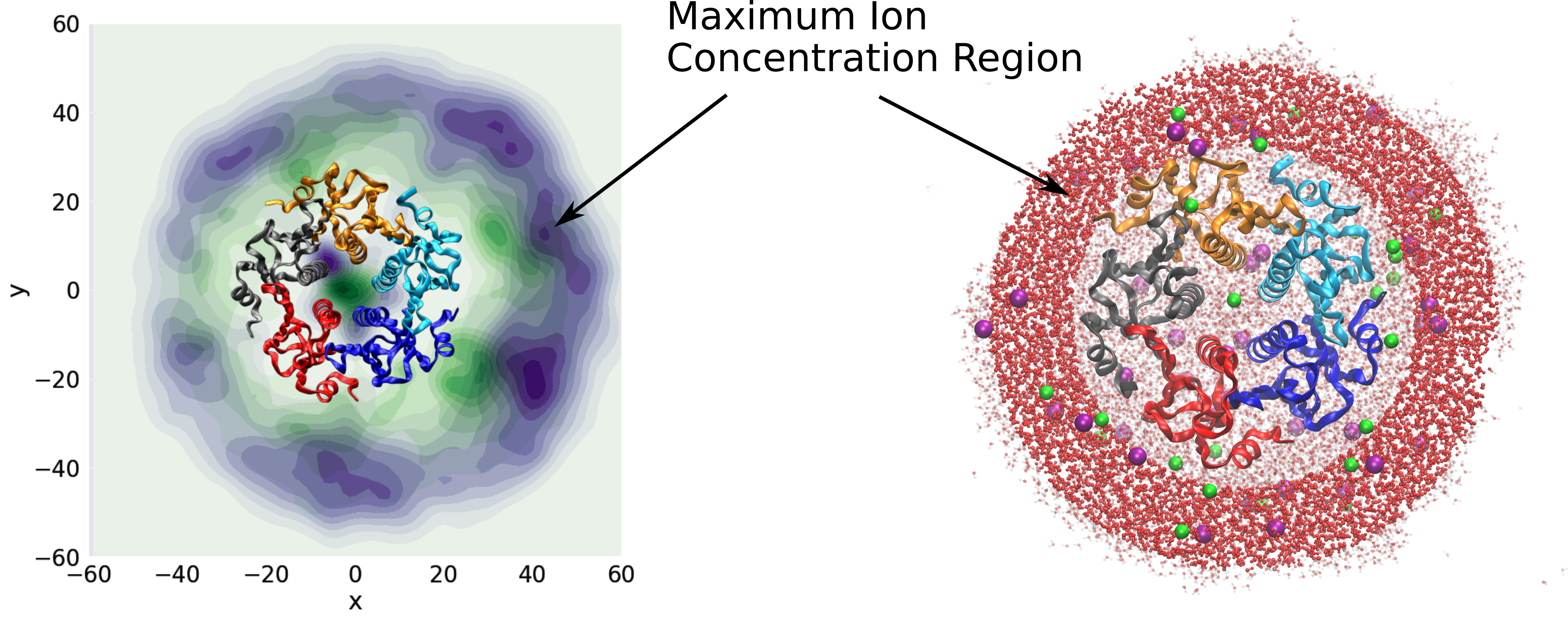
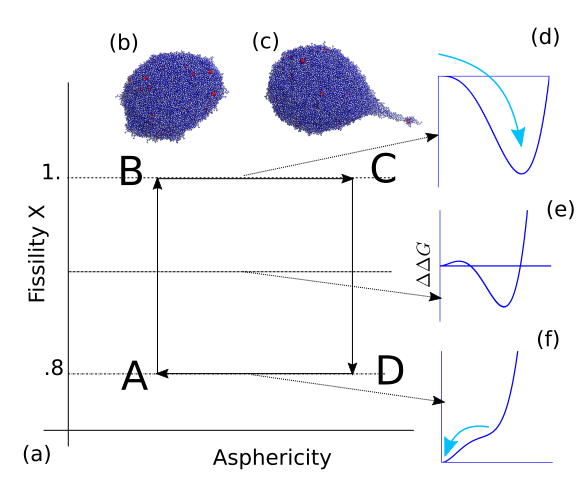
A direct outcome of this research is an improved understanding of the physical and chemical processes occurring in atmospheric aerosols, providing guidance for experimental design in mass spectrometry and for the interpretation of experimental data. One of our major achievements has been the elucidation of the mechanism of electrospray ionization, a widely used technique often coupled with mass spectrometry. Key findings include the formation of Rayleigh jets in charged droplets; the emergence of distinct chemical environments within conical protrusions on highly charged droplet surfaces; the formation of an excess-charge surface layer, which serves as the droplet analogue of the electrical double layer; and the mechanisms governing the charging of proteins and nucleic acids.
We are looking for passionate new PhD students and Postdocs to join the team (more info) !

News
10. December 2025
Han Nguyen defends MSc thesis entitled “Factors that determine Chemical Reactivity and Conformational Changes of a Peptide in Aqueous Droplets”
08. November 2025
Marko Voronych presents a poster entitled “Taylor cone formation in molecular clusters: A simulation-based assessment of electrostatic theory” at Chemical Physics Symposium
01. September 2025
Jihong Shi, Han Nguyen, and Mateo Pescador Arboleda present a poster “From sphere to cones: Structural instabilities and acidity at conical regions in trivalent metal ion nano-clusters” at the Lennard-Jones Centre-CECAM Meeting 2025
04. August 2025
S. Constas presents Chemistry in Supercooled Droplets at GRC Chemistry and Physics of Liquids
17. July 2025
S. Consta presents “Supercooling of droplets and formation of amorphous ice” at The McGill Molecular Science Mini-Meeting
17. June 2025
S. Consta presents “Multiscale modeling of electrostatic phenomena and chemical reactivity in nanoscale to microscale droplets” at Aqueous Systems: The Frontier and Beyond
15. June 2025
Han Nguyen and S. Consta present a poster entitled “Chemical reactivity in supercooled droplets” at CSC