Research
Chemistry in Small Volumes: from mesoscopic clusters to biological cells

Formation of droplets due to wave action.
Source: National Geographic
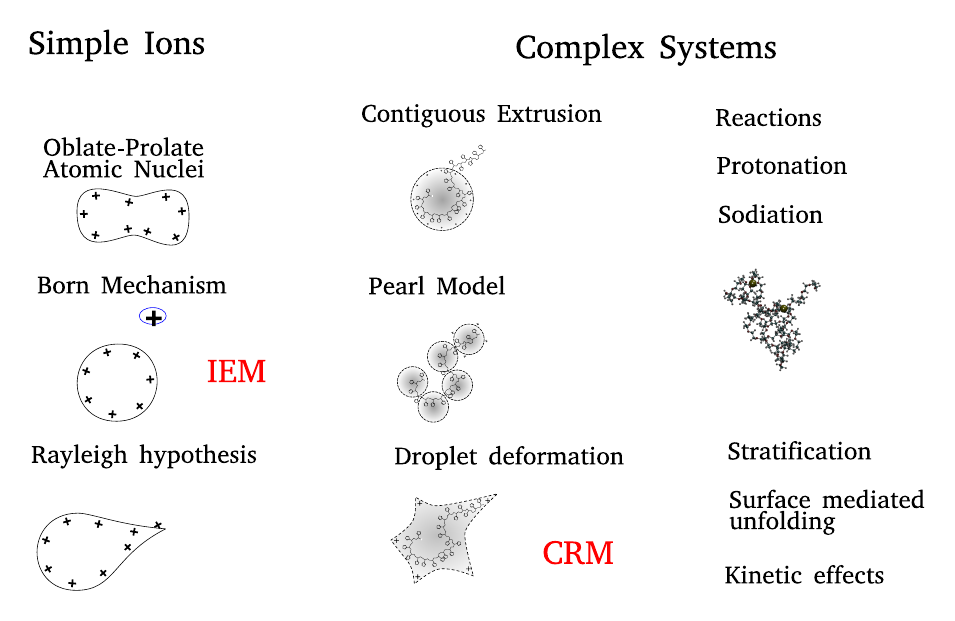
Droplets appear under different guises in many aspects of everyday life and technology. We observe them in naturally occurring atmospheric aerosols, in industrial and household sprays, as vesicles in nanofluidics and microfluidics, as emulsions, and as precursors of phase-separation processes. Droplets are often charged due to the presence of simple ions and macroions (e.g. nucleic acids, proteins, and other polyelectrolytes).
Electrospray-collision beam experiments [G. Cooks, Purdue University; R. N. Zare, Stanford] have demonstrated that droplets provide a distinct environment for chemical reactions—where certain reactions become orders of magnitude faster than in bulk. For this reason, chemistry in the small volume of droplets may be the future “beaker” of chemistry.
From another perspective, a cell and certain of its organelles share common features with a droplet. These features include confinement, crowding and shape fluctuations. Because of these commonalities, a droplet may be used as a model of a biological cell. Considering the broadness of the droplet world, discovering of the ion and macroion-droplet interactions is of central importance in atmospheric chemistry, biology, technology applications, analytical chemistry, microfluids and nanofluidics.
Atmospheric and Electrosprayed Aerosols
Aerosols are critical in climate, atmospheric visibility, air quality, and health. Despite decades of study, their chemistry remains poorly understood, particularly the reactions occurring on aerosol substrates, and the role of nucleation and growth. Aerosols remain one of the greatest uncertainties in predicting future climate change.
- Case study: The haze pollution in Beijing is a serious example of detrimental aerosol effects on health, visibility, and weather.
- Biological content: Aerosols may contain bacteria, fungal spores, pollen, viruses, and fragments of plants or animals. Their role in nucleation is still underexplored.
Using molecular modeling, we investigate:
- Ion interconversion in mesoscopic clusters (nano-drops)
- The role of biological material in nucleation and growth
- Methods to compute electric fields, shape fluctuations, surface tension, and dielectric constants in nano-drops
Scaling and extrapolation reveal how physical properties transition from nano-drops to micro-drops.
Key Publications:
- V. Kwan and S. Consta, J. Am. Soc. Mass Spectrometry (2020)
- V. Kwan and S. Consta, Chem. Phys. Lett. 746, 137238 (2020)
- V. Kwan, A. Malevanets, S. Consta, J. Phys. Chem. A 123(43): 9298–9310 (2019)
- A. Malevanets, S. Consta, J. Chem. Phys. 138(18): 184312 (2013)
- S. Consta, R. Kapral, J. Chem. Phys. 111: 10183–10191 (1999)
Star-Shaped Droplets
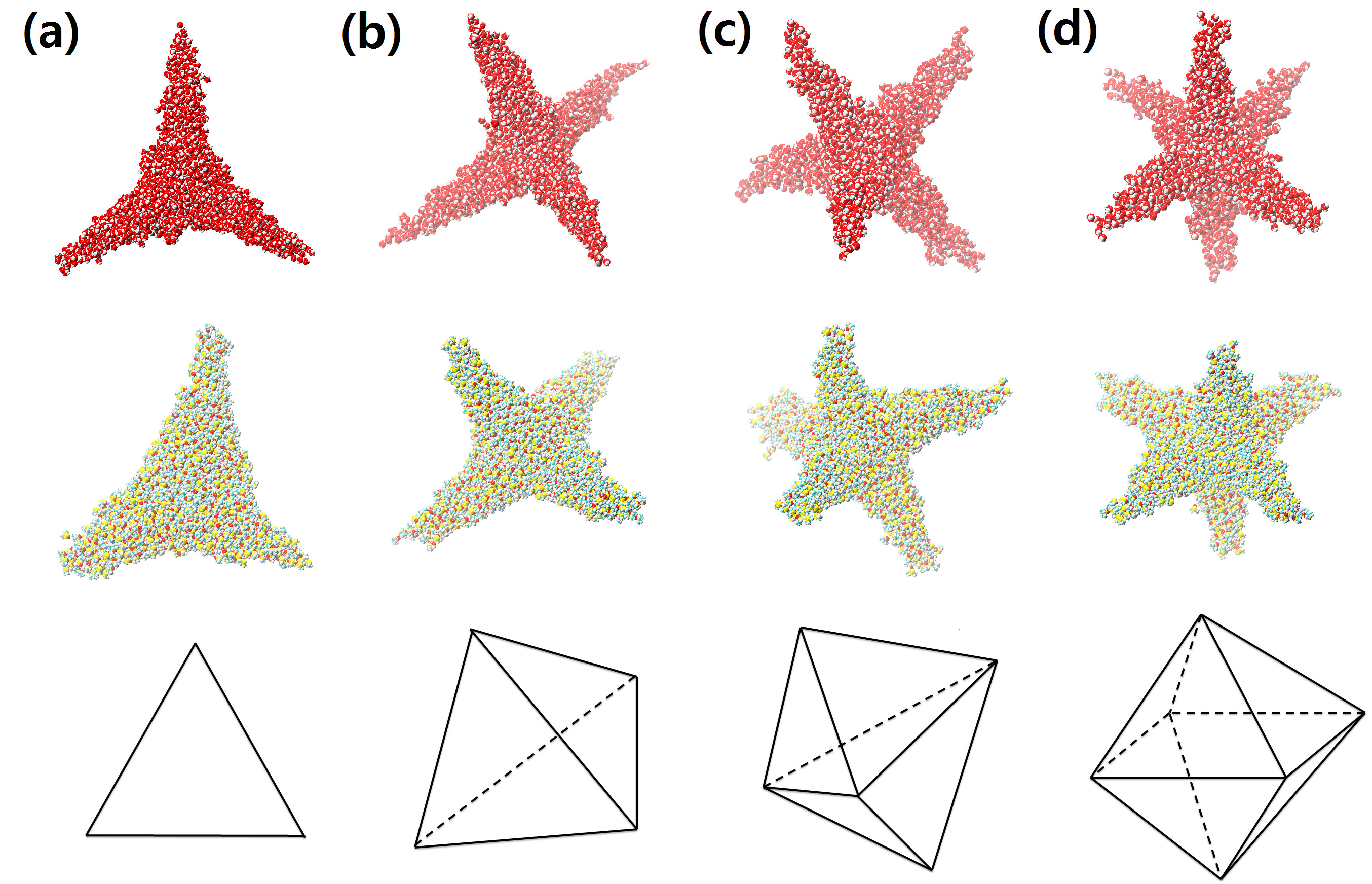
A key finding: droplets comprising a charged macroion and solvent can form “star-shaped” non-convex geometries. These shapes have the same morphology as branched colloidal nanocrystals. The fabrication of mono-disperse nano-crystal building blocks of complex geometry is a coveted goal in the assembly of functional nanostructures. Our discovery may open a new direction in production of solid highly non-convex colloids by adjusting the charge state of the central macroion. We analyze distinct features in the structure of the star-shaped nano-droplets using molecular and continuum modelling.
Publications:
- A. Malevanets et al., Mol. Phys. 116: 2892–2900 (2018)
- M. In Oh et al., Soft Matter 13: 8781–8795 (2017)
- M. Sharawy, S. Consta, J. Phys. Chem. A 120: 8871–8880 (2016)
- S. Consta, J. Phys. Chem. B 114(16): 5263–5268 (2010)
Dissociation Rates and Reaction Pathways
Two central questions in chemistry are:
- Free energy differences between species
- Relaxation rates toward equilibrium
Protein–ligand binding and macromolecular assemblies often occur on microsecond–millisecond timescales that are hard to capture with molecular dynamics. We apply first-principles approaches to determine reaction pathways and compare exact results with approximate techniques.
Publications:
- S. Consta, Theor. Chem. Acc. 116: 373–382 (2006)
- S. Consta, K. Mainer, W. Novak, J. Chem. Phys. 119(19): 10125 (2003)
- S. Consta, R. Kapral, J. Chem. Phys. 104(12): 4581–4590 (1996)
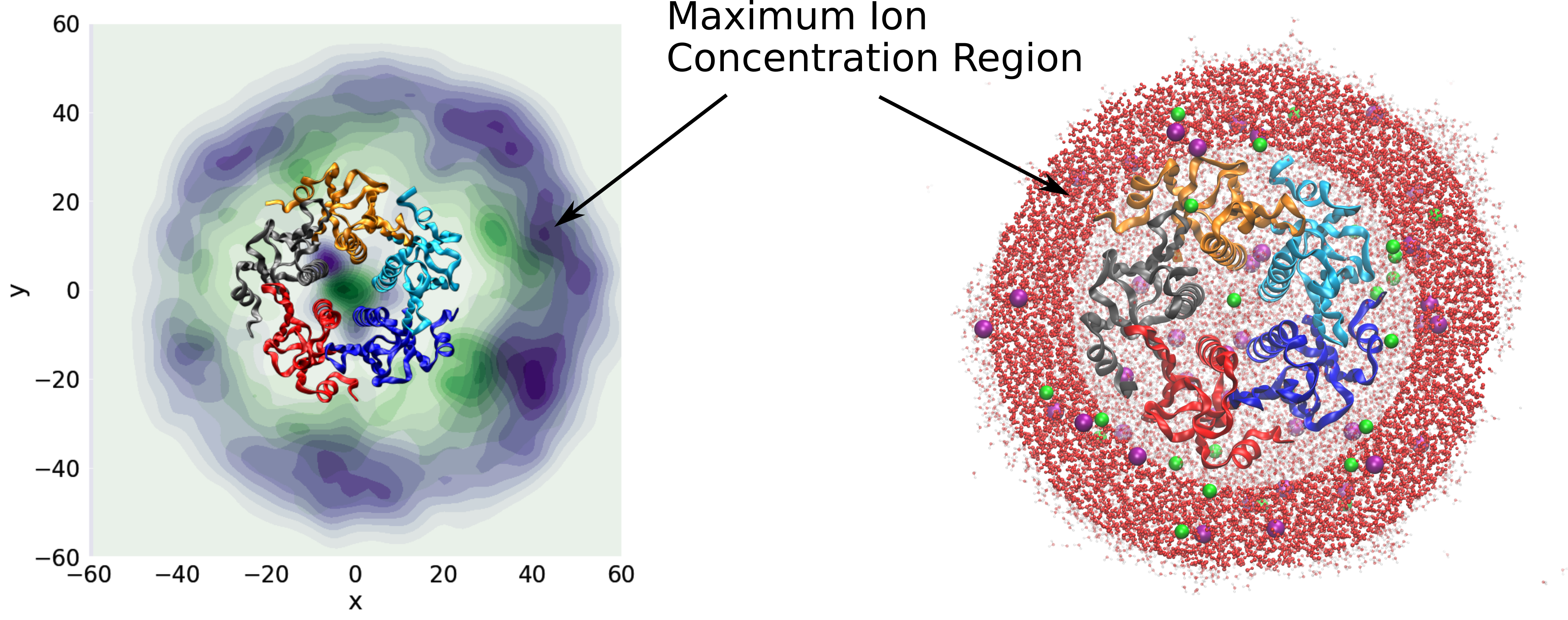
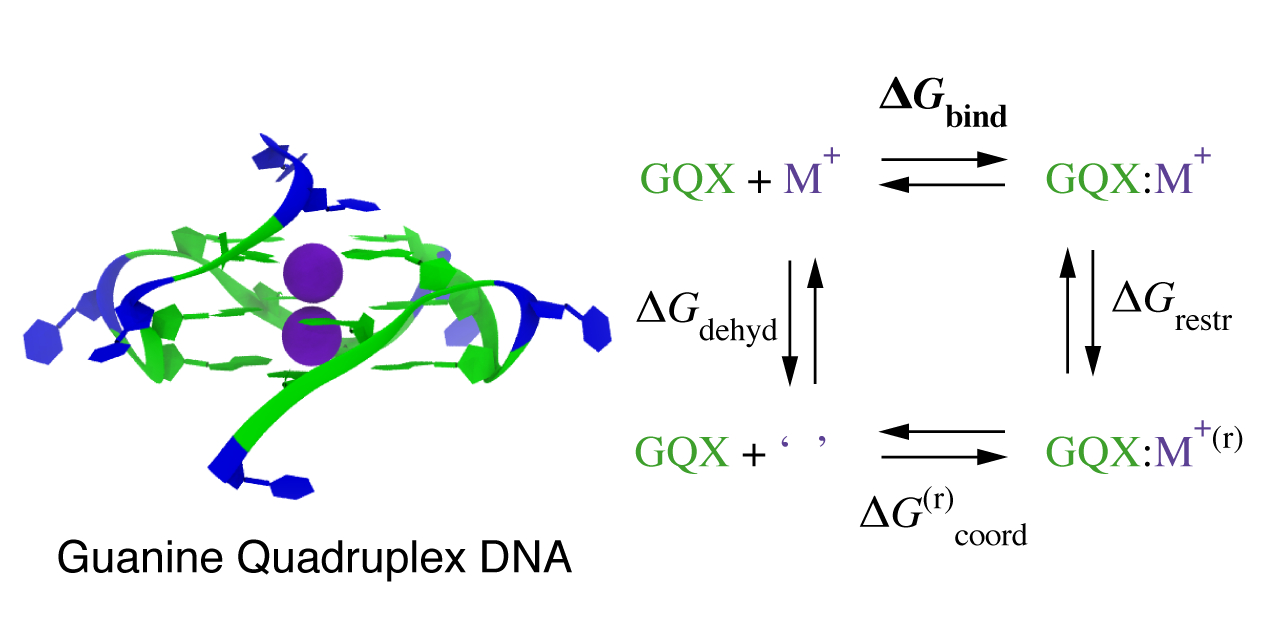
Macroion–Ion Interactions in Droplets and Bulk
We study the reactivity and ion distribution in charged droplets containing DNA, RNA, intrinsically disordered proteins, and assemblies. Results from atomistic modeling are compared with nonlinear Poisson–Boltzmann solvers developed in our group.
Publications (selected):
- M. Sharawy, S. Consta, J. Chem. Phys. 149: 225102 (2018)
- S. Consta et al., J. Phys. Chem. A 122(24): 5239–5250 (2018) (feature and cover article)
- S. Consta, M. In Oh, A. Malevanets, Chem. Phys. Lett.–Frontiers 663: 1–12 (2016)
- M. Sharawy, S. Consta, Phys. Chem. Chem. Phys. 17: 25550–25562 (2015)
- S. Consta, A. Malevanets, Phys. Rev. Lett. 109(14): 148301 (2012)
Protein–Protein and Protein–Ligand Interactions
Protein complexes provide diverse mechanisms for assembly. Accurate free-energy calculations are key to rational drug design and understanding biological function. We analyze protein stability in both droplets and bulk.
Publications:
- S. Consta et al., Anal. Chem. 89: 8192–8202 (2017) (Perspective)
- M. In Oh, S. Consta, Phys. Chem. Chem. Phys. 19: 31965–31981 (2017)
- M. In Oh, S. Consta, J. Phys. Chem. Lett. 8: 80–85 (2017)