Introduction to Apoptosis
& Efferocytosis
Apoptosis,
the controlled demolition of old, unneeded, infected or damaged cells,
is fundamental to homeostasis and immunity. Each day billions of cells
in our body undergo apoptosis, wherein the cellular contents of dying
cells are degraded and packaged into membrane bound vesicles termed
apoptotic bodies. Apoptotic bodies serve a dual purpose: they prevent
the spillage of cellular contents into the extracellular milieu, while
simultaneously packaging cell contents into particles small enough to
be internalized by professional phagocytes.
The clearance of apoptotic bodies by
phagocytes – termed efferocytosis
– is required for tissue homeostasis, with failure to clear these
particles leading to inflammation, autoimmunity and neurodegenerative
diseases. If not cleared promptly, apoptotic bodies rupture and release
their contents in a process termed secondary necrosis. Because these
intracellular contents include pro-inflammatory substances such as
nucleotides (ATP, UTP), secondary necrosis promotes inflammation.
Indeed, the defective removal of apoptotic cells is an initiating event
in inflammatory disorders such as atherosclerosis.
While it is unclear if secondary necrosis drives autoimmunity,
it is well established that the presentation of antigens derived from
apoptotic cells plays a central role in maintaining self-tolerance,
with failures in this system leading to autoimmunity. The regulation of
apoptosis and the subsequent clearance of apoptotic cells also
contributes to immunity against infectious agents such as viruses and
intracellular bacteria. Efferocytosis of apoptotic bodies released by
infected cells allows for the processing and presentation of
intracellular pathogen-derived antigens by professional phagocytes.
These phagocytes then transport these normally sequestered antigens to
lymphatic tissues, where the antigens are the presented on MHC II, thus
driving the formation of adaptive immunity.
Despite the obvious importance of efferocytosis, little is known about the process itself. Efferocytosis is a three step process, consisting of an initial recognition of the apoptotic body, internalization of the apoptotic body by a phagocyte, and finally, destruction of the apoptotic body. Research in the Heit lab focuses on the signalling which regulates these processes, with a focus on the receptors that bind apoptotic bodies and the signalling these receptors induce.
Efferocytosis
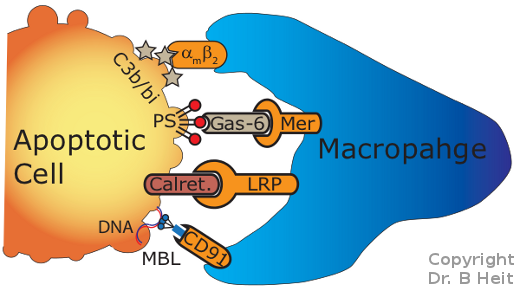
Efferocytosis occurs when receptors on
phagocytes
such as macrophages recognize "eat me" signals displayed by
dying (apoptotic) cells (Figure 1). These
eat-me signals are recognized by specialized receptors on the
macrophage. When engaged, these receptors induce a signaling
pathway which controls the consumption of the apoptotic cell by the
phagocyte.
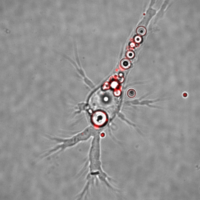
Following the uptake of the apoptotic
body, it must be degraded by the phagocyte (Figure 2).
It is thought that this process occurs through the same phagocytic
mechanism used by phagocytes to destroy bacteria and other
pathogens. However, evidence developed in our lab suggests that
this is not the case, and instead, a unique degradative pathway appears
to be involved.
Failures in the efferocytic process appears to underly many human diseases. Inappropriate processing of apoptotic cells may cause the phagocyte to display antigens derived form the apoptotic cell to the immune system. This inappropriate presentation of self-antigens leads to autoimmune diseases such as lupus, multiple sclerosis and type I diabetes. By understanding the normal processing verses abnormal processing of apoptotic-cell derived antigens, we hope to find new therapies to reverse or prevent autoimmune diseases.
Efferocytic
failures also appear to occur during
atherosclerosis,
which leads to heart
attacks and stroke.
It is normal for the cells in our hearts to take up "bad"
cholesterol. This stresses the cells, leading them to die via
apoptosis. Normally these dead cells are cleared by
efferocytosis, but in some individuals these apoptotic cells are not
removed, leading to the accumulation of dead and dying cells beneath
the blood vessels of the heart. This accumulation is termed an
atheroma
or atherosclerotic plaque, and if this atheroma grows too large it
can rupture, leading to blood clots. If these clots lodge in
the heart or brain, the patient will respectively experience an
ischemic stroke or heart attack. By determining why the
efferocytic process fails in atherosclerotic patients we may be able
to find ways to reverse or prevent atherosclerosis.
Phagocyte Membrane Biology
The plasma membrane
is the sole barrier separating the cellular machinery from the
extracellular environment. This imbues the plasma membrane with
critical roles in modulating signal transduction, transport and
secretion. In efferocytois, the plasma membrane acts as both the
surface that contains the efferocytic receptors, and is the surface of
the apoptotic cell recognized by these receptors. Its long been
thought that the plasma membrane was comprised of a "sea" of lipids in
which proteins floated freely. Recent evidence has shattered
these assumptions, demonstrating that cellular membranes are segmented
such that most proteins in the membrane are confined to small regions
(80-200 nm) of the membrane termed corrals. Furthermore, the liquid
portion of the plasma membrane is divided into numerous microdomains –
small (<100 nm) "islands" in which specific membrane lipids and
proteins are concentrated.
Microdomain "Islands": The number, type and size of microdomain "islands" is unknown, but at a minimum four types exist - lipid rafts, tetraspanin-enriched microdomains (TEMs), protein-stabilized microdomains (PSMs) and glycosphingolipid- enriched microdomains (GEMs). The role of these microdomains is unknown, but we hypothesize
that these islands act to assemble the receptors which recognize
apoptotic cells into preformed complexes along with their signaling
molceules and co-receptors. This enhances the function of
efferocytic receptors by ensuring they remain associated with the
machinery which allows them to work, while at the same time preventing
accidental triggering of the receptors by segregating them into
complexes which are too small on their own to signal. Upon
engaging an apoptotic cell these domains would cluster, activating the
receptors.
Corrals: Further complicating the substructure of the plasma membrane are corrals – “fences” of an unknown composition that partitions the plasma membrane into numerous sub-regions. Within corrals membrane proteins diffuse freely, but escape from corrals is limited. It is not clear what forms these corrals, but it is thought that areas of low cytoskeleton density below the membrane may create these corrals, with the barriers between corrals comprised of higher density cytoskeleton. We believe these corrals serve two very important purposes - firstly, we hypothesize that corrals act to prevent spontaneous signaling through phagocytic receptors by limiting the ability of membrane proteins to spontaneously coalesce. Secondly, we hypothesize that interactions between these corrals and membrane proteins are required for phagocytes to generate forces, such as those required to internalize an apoptotic cell.
Research in the Heit lab addresses these three hypotheses, using phagocytes and apoptotic cells as a model system. Importantly, clinically relevant factors such as hypercholesterolemia
can alter the structure and formation of microdomains and corrals,
suggesting that atherosclerosis-associated efferocytic defects may stem
directly from membrane defects caused by excess cholesterol.
Also from this web page: