Technologies Used in the Laboratory
BD FACSCanto™ II
Flow cytometry is a powerful method for studying many aspects of immune responses, and modern immunology laboratories depend heavily on flow cytometers for their daily research operation. The BD FACSCanto™ II system, which was provided to us through a generous Leaders Opportunity Fund from Canada Foundation for Innovation (CFI), is a state-of-the-art benchtop flow cytometer that allows for accurate multi-parameter (up to eight fluorochrome-based colors plus forward scatter and side scatter) analysis of various cell types. These include, but are not limited to, antigen-specific CD8+ T cells and immunoregulatory invariant natural killer T (iNKT) cells that are a subject of intense investigation in our laboratory. Our flow cytometer is available to our lab members and collaborators only.
C.T.L. ImmunoSpot® S5 UV Analyzer

This cutting-edge and fully automated analyzer is designed for high throughput, yet extremely precise scanning and analysis of data generated in enzyme-linked immunospot (ELISPOT) and fluorescence-based spot (FLUOROSPOT) assays. These techniques enable ultrasensitive detection of rare immune cell populations (e.g., antigen-specific B or T lymphocytes) based on antibodies, cytokines or other effector molecules they secrete. The Analyzer’s multiple revolving filters permit automated switching between several wavelengths. One filter is reserved for conventional ELISPOT assays and the others can detect different colors of fluorescence. The “autocentering” feature of the Analyzer allows our lab members to define safety margins of microplate wells and capture 100% of the well bottom surface readily and accurately. The visualization and counting of single fluorescent cells are achieved by the high resolution optics and digital color camera of the Analyzer.
|
Applied Biosystems® StepOnePlus™ Real-Time PCR System

For our gene expression analyses at the mRNA level, our laboratory is equipped with a user-friendly StepOnePlus polymerase chain reaction (PCR) machine. This system measures amplification as it occurs, cycle by cycle, allowing for precise and quantitative measurements during the exponential phase of PCR.
|
PerkinElmer 1470 Automated Gamma Counter

Cytotoxic lymphocytes, namely CD8+ T cells, NK cells and NKT cells, use multiple mechanisms and pathways to destroy their targets such as virus-infected cells and tumor cells. Our laboratory studies their cytotoxic effector function and lytic mediators and mechanisms. Cell-mediated cytotoxicity is measured by various methods including the traditional but still powerful 51Cr release assays. In these assays, target cells are labeled with 51Cr before they are co-incubated with killer cells. Cytotoxicity is then measured by quantification of the 51Cr activity of culture supernatant samples using a γ counter. Scientists in the Haeryfar lab receive ample training and become fully certified before they can work with radioisotopes such as 51Cr.
|
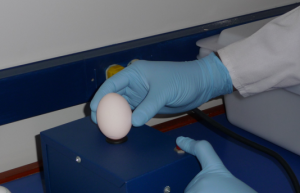
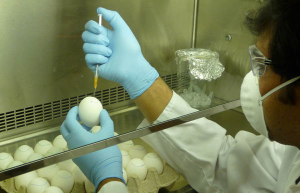
Egg Incubator
|
For our in vivo investigations of antiviral immunity and for our flu vaccination studies, we propagate influenza A viruses within the allantoic cavity of chicken eggs. Candling, inoculation and incubation of eggs in a specialized chamber are some of the steps in this process. The excellent viral yield from chicken eggs has led to their widespread usage in influenza research and vaccine production. In fact, the vast majority of influenza vaccines – both inactivated and live – are produced in chicken eggs.
|