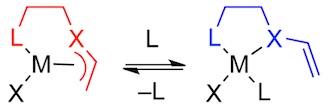Projects
We target the development of clean, efficient and robust catalysts for sustainable chemical transformations. These advances are critical to decreasing our energy and waste footprint in both the academic and industrial chemical realms.
As small molecule chemists, we strive to achieve a detailed and accurate understanding of chemical structure and mechanism. We closely analyze how molecules like water and dioxygen interact with metal complexes. These insights inform how we design catalysts to exploit clean and abundant feedstocks to make high-value organics. Specific catalyst design characteristics that we exploit include dynamic and/or coorperative ligand structures.
Members of the research team each have a specific project, but communication amongst members is critical to the problem solving process. Projects vary in type with some being mostly synthetic while others involve catalytic testing and/or mechanistic analysis. All projects use a variety of analytical tools including: NMR, Mass Spectrometry, GC-MS, Crystallography, UV-vis and IR.
Dynamic Ligand Coordination to Maximize Catalyst Lifetimes
Low catalyst performance is often attributed to competitive decomposition that proceeds via low-coordinate catalyst intermediates. Such species can be stabilized, and decomposition circumvented, with ligands that dynamically increase their coordination number to the metal. We are developing a new ligand family that dynamically changes its coordination to the metal through a change in ligand hapticity. These ligands are being used with a variety of metal catalysts to maximize lifetime.
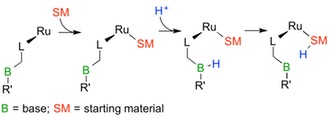
Proton-Transfer (PT) Catalysts for Atom-Economic Transformations
Proton-transfer steps to or from the metal-bound organic intermediate are important in many catalytic reactions. These steps can be dramatically accellerated if the supporting ligand is modified to include a functional group that mediates the PT steps in an intramolecular fashion. We are investigating a number of reactions that involve such PT steps including, hydration, cyclization and dehyrogenation reactions. A key focus of our work includes a systematic study of the optimal PT properties of the ligand.
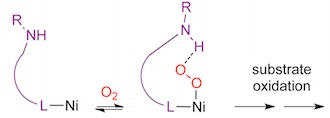
Direct Oxidation of C-H Bonds
The number of steps required to reach a target compound is a major contributor to synthetic inefficiency. Net oxidations are step inefficient processes where a typical conversion of a C-H to C-O moiety takes three or more steps. We are investigating the ability of Ni(II) to act as catalysts for one-step aerobic C-H oxidation reactions. Hydrogen-bond donor groups are incorporated to stabilize reactive intermediates that aids in improved catalyst performance (i.e. selectivity and activity).