| ||||
|
Research Spanning Peptides and Oligonucleotide Chemistries
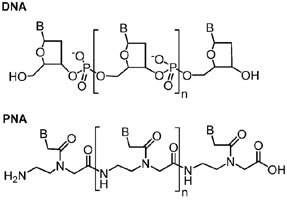
Our principle research activities involve the application of organic synthesis in the field of bio-organic chemistry. We specialize in oligonucleotide analogs and our work is focused on peptide nucleic acid (PNA) and nucleobase-modified DNA and RNA with particular interest in fluorescent analogs. PNA and DNA/RNA Research Areas The term Peptide Nucleic Acid (PNA) now refers to a large number of nucleic acid mimics based on a synthetic amino acid monomer unit. The original design, as shown below in comparison to DNA, was disclosed by Peter Nielsen and coworkers in the early 1990's. Since that time a great deal of work has been done in the chemistry and biochemical/biological properties and applications of PNA. Our research program has mostly focused on the chemistry and effects of various nucleobase modifications on the properties of PNAs. As well, we are interested in similar modifications to DNA and RNA.
Nucleobase Modifications and Luminescent Nucleobases.
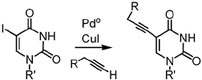
The pyrimidine nucleobases uracil and cytosine are particularly attractive candidates for modification in PNA because of the wealth of established chemistry for them. For some time, we have pursued is via the cross-coupling between terminal alkynes and a halogenated nucleobase, either uracil or cytosine.
R.H.E Hudson and J.M. Moszynski “A facile synthesis of fluorophores based on 5-phenylethynyluracils” Synlett, 2006, 18, 2997-3000. These studies lead us to the investigation of 5-alkynylpyrimidines and their cyclization to form fluorescent bicyclic nucleobases. We have found that the luminescent properties of these compounds varies with the nature of the substituent on the 5-membered ring ("R") and are currently investigating their use as DNA probes.
R.H.E. Hudson, A.K. Dambenieks, R.D. Viirre “Fluorescent 7-deazapurine derivatives from 5-iodocytosine via a tandem cross-coupling/annulation reaction” Synlett 2004, 13, 2400-2402.
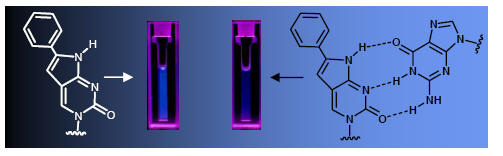
R.H.E. Hudson and A.K. Dambenieks “Synthesis of N1-unsubstituted 5-alkynylcytosine and derivatives thereof” Heterocycles, 2006, 68(7), 1325 - 1328. In DNA oligomers, 6-phenylpyrrolocytidine can selectively yield fluorometric detection of guanosine-containing sequences in complementary DNA. It is a very robust system, as the fluorescence is selectively quenched by guanosine but not mismatched bases.
R.H.E. Hudson and A. Ghorbani-Choghamarani “Selective Fluorometric Detection of Guanosine-Containing Sequences by 6-Phenyl-pyrrolocytidine in DNA” Synlett, 2007, 6, 870-873.
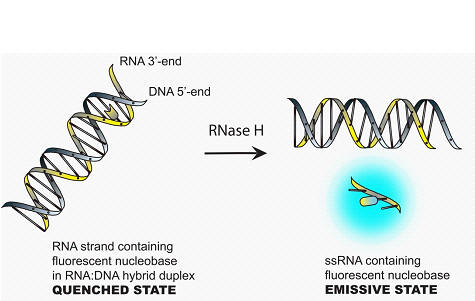
In RNA, 6-phenylpyrrolocytidine (PhpC) acts as a single-label molecular beacon for the observation of RNase H activity associated with HIV-RT. An embedded PhpC is quenched, however after cleavage by the enzyme, a small fluorescent fragment is released signalling the cleavage event. The PhpC-containing heteroduplex is a better tolerated substrate than the conventional molecular beacon construct featuring a fluorescein labelled RNA and a quencher (DABCYL) labelled DNA strand and produces full cleavage in just over 20 minutes versus about 2 hours for the F-Q system. This allowed us to develop a rapid and sensitive assay for RNase H acitivity and inhibitors thereof.
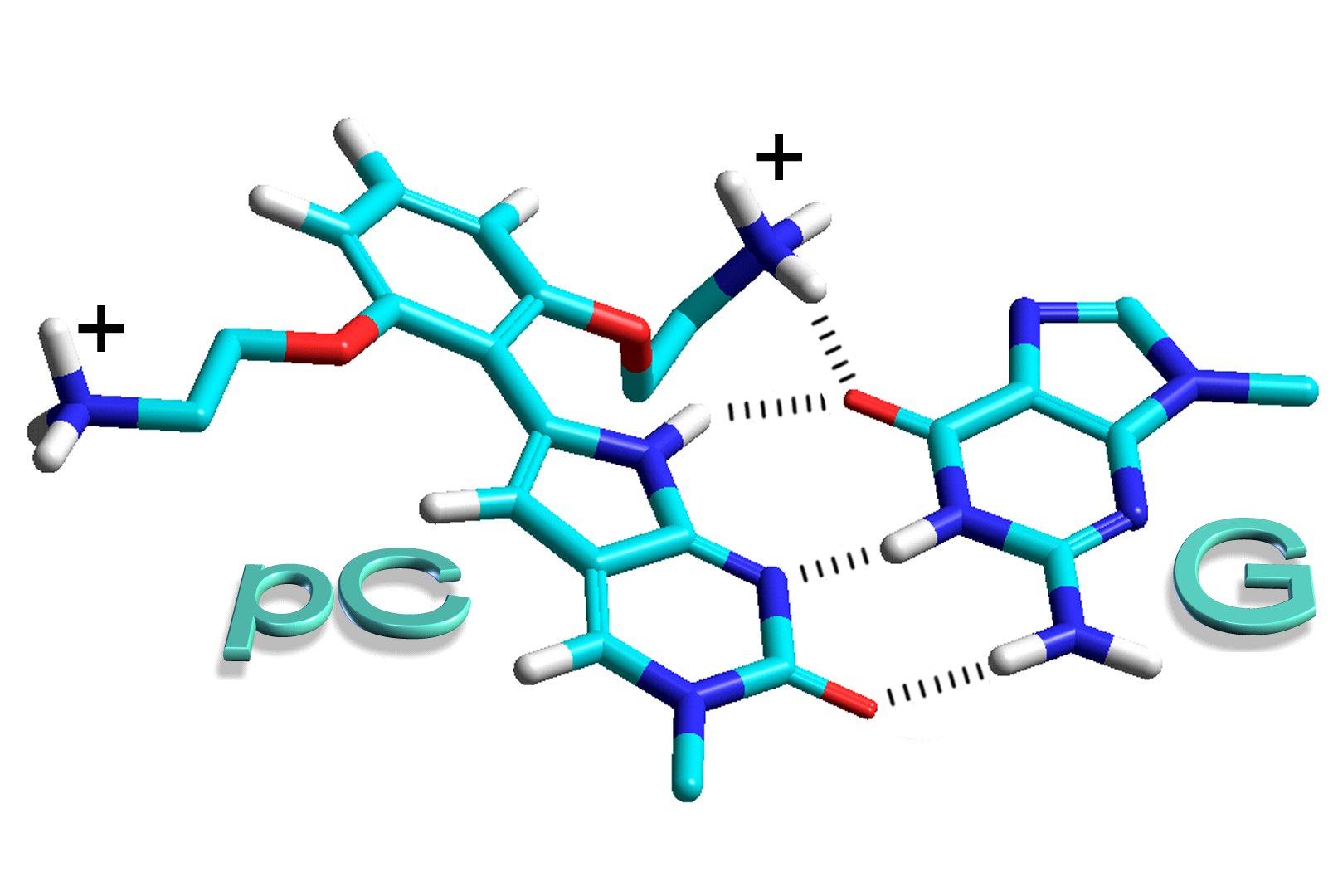
Alexander S. Wahba, Abbasali Esmaeili, Masad J. Damha† and Robert H. E. Hudson† “A Single-Label Phenylpyrrolocytidine Provides a Molecular Beacon-Like Response Reporting HIV-1 RT RNase H Activity”, Nucleic Acids Research, 2010, 38(3), 1048-1056. Substitution on the phenyl ring leads to pyrrolocytosines that interact more strongly with guanosine through a tether designed to form a 4th Hydrogen bond, when incorporated into PNA oligomers (bis-ortho substituted phenylpyrrolocytosine - the BoPhpC monomer). These modified pCs also fluorimetrically respond to hybridization.
F. Wojciechowski and R.H.E. Hudson "Fluorescence and Hybridization Properties of Peptide Nucleic Acid Containing a Substituted Phenylpyrrolocytosine Designed to Engage Guanine with an Additional H-Bond” Journal of the American Chemical Society, 2008, 130(38), 12574-12575.
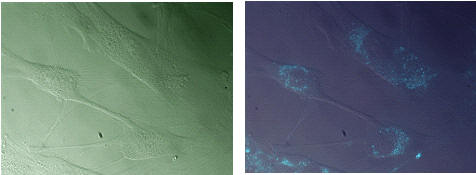
The intrinsic fluorescence of BoPhpC can be used to visualization cellular uptake of PNA Based antisense agents (targeted to Huntingtin mRNA)
Differential interference contrast
BoPhpC-PNA fluorecence J. Hua, D.W. Dodd, R.H.E. Hudson and D.R. Corey Bioorganic and Medicinal Chemistry Letters, 2009, 19, 6181-6184.
The PhpC framework also serves as a convenient scaffold for variation. We have shown that the binding properties can be optimized by varying the position, length and nature of the pendant group on the arm.
Filip Wojciechowski and Robert H.E. Hudson “Peptide Nucleic Acid Containing a meta-Substituted Phenylpyrrolocytosine Exhibits a Fluorescence Response and Increased Binding Affinity Towards RNA” Organic Letters, 2009, 11(21), 4878–4881.
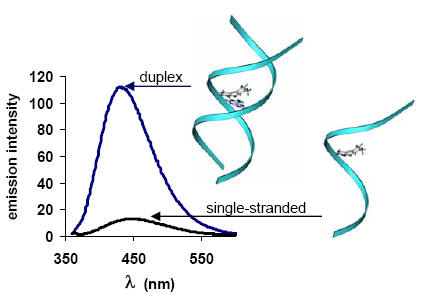
Oligodeoxynucleotides incorporating structurally simple 5-alkynyl-2 -deoxyuridines fluorometrically respond to hybridization. The principle of selective recognition of adenosine by 5-alkynyluridines versus the known fluorescent, bicyclic derivative furanouracil is shown. Cyclization is not required for fluorescence given the proper choice of R', such as phenyl. 5-Phenylethynyl-2'-deoxyuridine containing oligomers acts as "light probes" for complementary sequences, up to a 6-fold increase in fluorescence may be observed.
R.H.E. Hudson† and A. Ghorbani Choghamarani “Oligonucleotides Incorporating Structurally Simple 5-Alkynyl-2’-Deoxyuridines Fluorometrically Respond to Hybridization” Organic and Biomolecular Chemistry, 2007, 5(12), 1845-1848.
Medical Imaging.
We are involved in collaboration with coworkers at the Robarts Research Institute focused on the synthesis of materials for optical and magnetic resonance imaging for potential clinical applications.
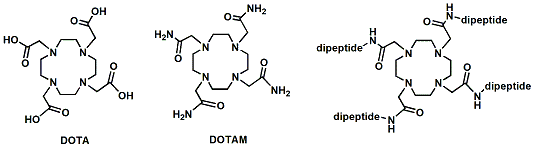
We have recently developed peptide-decorated lanthanide metal chelators as MRI contrast agents that operate on the basis of paramagnetic chemical exchange saturation transfer (PARACEST). These ligands are similar to the well-known DOTA and DOTAM ligands.
Filip Wojciechowski, Mojmir Suchy, Alex X. Li, Hassan Azab, Robert Bartha and Robert H.E. Hudson† “A Robust and Convergent Synthesis of Dipeptide-Cyclen Conjugates as Chelators for Lanthanide Ions: New Paracest MRI Agents” Bioconjugate Chemistry, 2007, 18, 1625-1636.
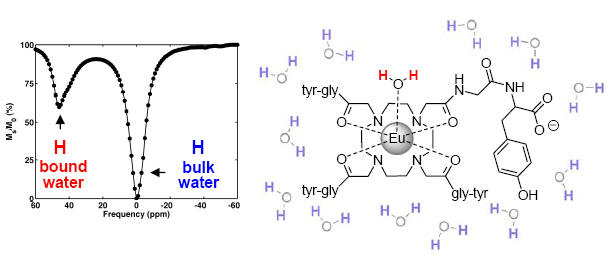
A PARACEST spectrum: the bound water signal is separated from the bulk water signal. The intense signal, approx. 45% under the experimental conditions and at physiologic pH and temperature may be used to generate contrast in PARACEST-based MR imaging.
Mojmír Suchý,Alex X. Li, Robert Bartha and Robert H.E. Hudson† “Analogs of Eu3+ DOTAM-Gly-Phe-OH and Tm3+ DOTAM-Gly-Lys-OH: Synthesis and Magnetic Properties of Potential PARACEST MRI Contrast Agents” Bioorganic and Medicinal Chemistry, 2008, 16(11), 6156-6166. Alex X. Li, Filip Wojciechowski, Mojmir Suchy, Robert H.E. Hudson, Ravi Menon and Robert Bartha† “A Sensitive PARACEST Contrast Agent for In-vivo Temperature MRI: Eu3+-DOTAM-Gly-Phe” Magnetic Resonance in Medicine, 2008, 59, 374-381.
Synthesis of Cystamine Modified Eu3+ DOTAM-Gly-Phe-OH: A Conjugation Ready Temperature Sensitive MRI Contrast Agent. Modification of the ligand yet retention of the most desirable PARACEST effects has been achieved. 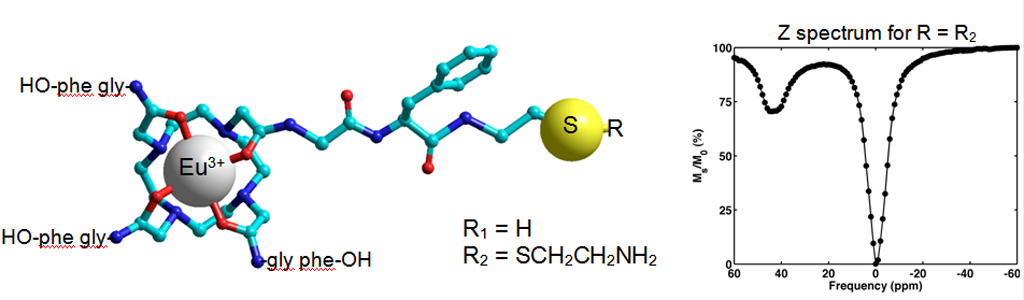
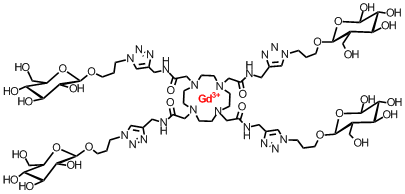
We have also extended our studies to "click" derivatization to produce ligand diversity. Where a large variety of decorated chelators may be synthesized - such as the glucose-labelled one shown below:
Mark Milne, Kirby Chicas, Alex Li, Robert Barthab and
Robert H.E. Hudson† “ParaCEST MRI Contrast Agents Capable of
Derivatization via “Click” Chemistry” Organic and Biomolecular Chemistry,
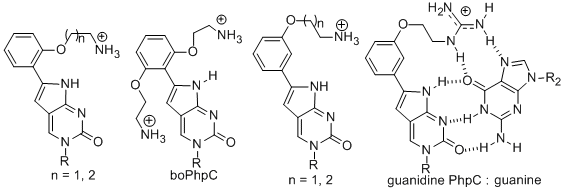
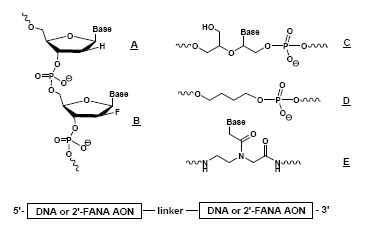
2011. DOI:10.1039/C1OB06162C. Other Applications of PNAs. Using chimeric, modified DNA/RNA and PNA to examine the structural requirements for substrate recognition of HIV RNAse H enzyme. Chemical structures used:
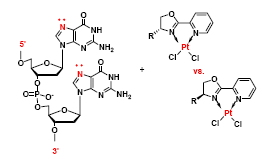
Pt-based Anticancer Agents. In collaboration with our Inorganic colleagues (Prof. Westcott, MTA and Prof. Jones, UWO), we have investigated the reactivity of novel cis-dichloroplatinum complexes with DNA. The archetypal compound, cisplatin (cis-diammineplatinum dichloride) is a very successful and widely used anticancer drug, but has significant side-effects and limitations - thus the search for new compounds with similar action.
L.G. Nikolcheva, C.M. Vogels, R.A. Stefan, H.A. Darwish, S.J. Duffy, R.J Ireland, A. Decken, R.H.E. Hudson†, S.A Westcott† Canadian Journal of Chemistry 2003, 81 (4), 269-274.
A.S. King, L.G. Nikolcheva, C.R. Graves, A. Kaminski, C.M. Vogels, R.H.E. Hudson, R.J. Ireland, S.J. Duffy, S.A. Westcott† Canadian Journal of Chemistry 2002, 80, 1217-1222.
Find out more about our group,
facilities,
research and publications by following these links.
| ||||
| We gratefully acknowledge funding from: | ||||
 |
|
|||
|
Department of Chemistry Chemistry Building Laboratory 219A The University of Western Ontario
Tel: 519-661-2111x86349 Fax: 519-661-3022 Email: Robert.Hudson
|
||||