Research Summary
Synthetic chemistry is the process of rationally constructing molecules through
a series of chemical reactions. Organic synthesis, historically has been concerned with the construction of molecules comprised mainly of carbon, hydrogen, oxygen and nitrogen and has been normally linked to molecules of nature which often posses biological activity. Inorganic synthesis is normally associated with molecular architectures which do not have carbon as the central element and have been most closely associated with materials chemistry or catalysis.
Of late, however, there has been a renaissance which has destroyed this constrictive and artificial barrier and many in the field prefer "chemical synthesis" to describe their research.The reasons for doing chemical synthesis are manifold but most include the desire to improve quality of life. This may be done by synthesizing new materials for electronic devices which make our lives so much easier or by preparing new materials for construction which are lighter and stronger than ever before. Of interest to our research group, however, is the desire to prepare molecules which improve quality of life from a healthcare standpoint - that is molecules of pharmaceutical interest. Our field of research
then is synthetic organic chemistry directed towards the synthesis of biologically active molecules and the development of new strategies and
reactions which will be of use to the medicinal chemist.
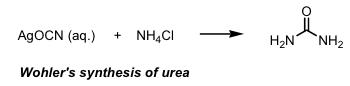 The birth of organic synthesis is usually considered the
synthesis of the natural product urea (NH2CONH2) by Wöhler in 1828. On treating silver cyanate with ammonium chloride solution he obtained a white crystalline material which proved identical to urea obtained from urine.
The birth of organic synthesis is usually considered the
synthesis of the natural product urea (NH2CONH2) by Wöhler in 1828. On treating silver cyanate with ammonium chloride solution he obtained a white crystalline material which proved identical to urea obtained from urine.
During this period, naturally occurring molecules were thought of as privileged structures with their own life force (vitalism). It was not thought possible to prepare a "living" molecule from non living ones. Since that time chemists have developed the art and science of organic synthesis to the stage that hugely complex naturally occurring molecules now succumb to the skill and tenacity of the synthetic chemist. I have often described organic synthesis to my family as playing with molecular tinker toys where the atoms which make up the molecules are the wooden disks and the sticks are the chemical bonds. The problem is that we cannot grab the atoms and attach them together by hand - instead we use chemical reactions to do the same thing. The connection of a single atom to another (one wooden disk to another) requires a chemical reaction. The more complex the target molecule the more difficult and lengthy the task. An intimate understanding of what can and cannot yet be accomplished as well as a large selection of chemical reactions is of huge importance to us in our goal of complex molecule synthesis.
In Our Group
Our research group has two main goals: 1) we develop new and fundamentally useful reactions which add to the arsenal of the synthetic organic chemist. These tools are used by the synthetic community both academic and industrial to prepare molecules of theoretical and commercial importance, and 2) we use our newly developed reactions and strategies to prepare such targeted molecules. By doing so, we showcase our synthetic methods as well as develop new synthetic strategies for the construction of complex molecular architectures. Below is a case in point.
An Example of Our Research
The development of a new reaction and application to the synthesis of nakadomarin A:
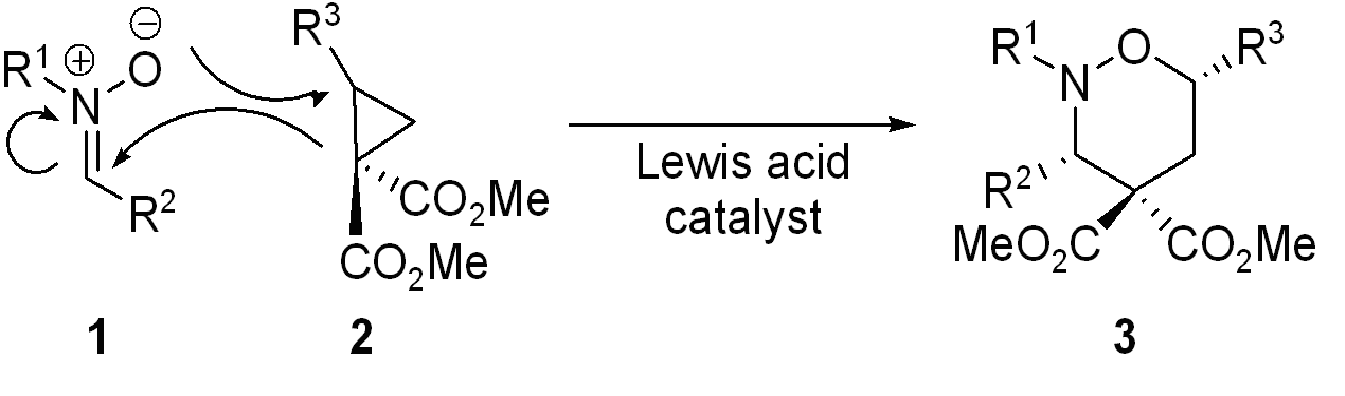
Recently we have described the reaction of nitrones with cyclopropanes in what we have termed a homo-3+2-dipolar cycloaddition In this reaction (Scheme 1) the combination of a nitrone 1 with a 1,1-cyclopropanediester 2 under the influence of a Lewis acid (most suitably a lanthanide triflate) results in the smooth formation of a tetrahydro-1,2-oxazine 3 with groups R2 and R3 on the same side of the six-membered ring.
Scheme 1: A new cycloaddition reaction
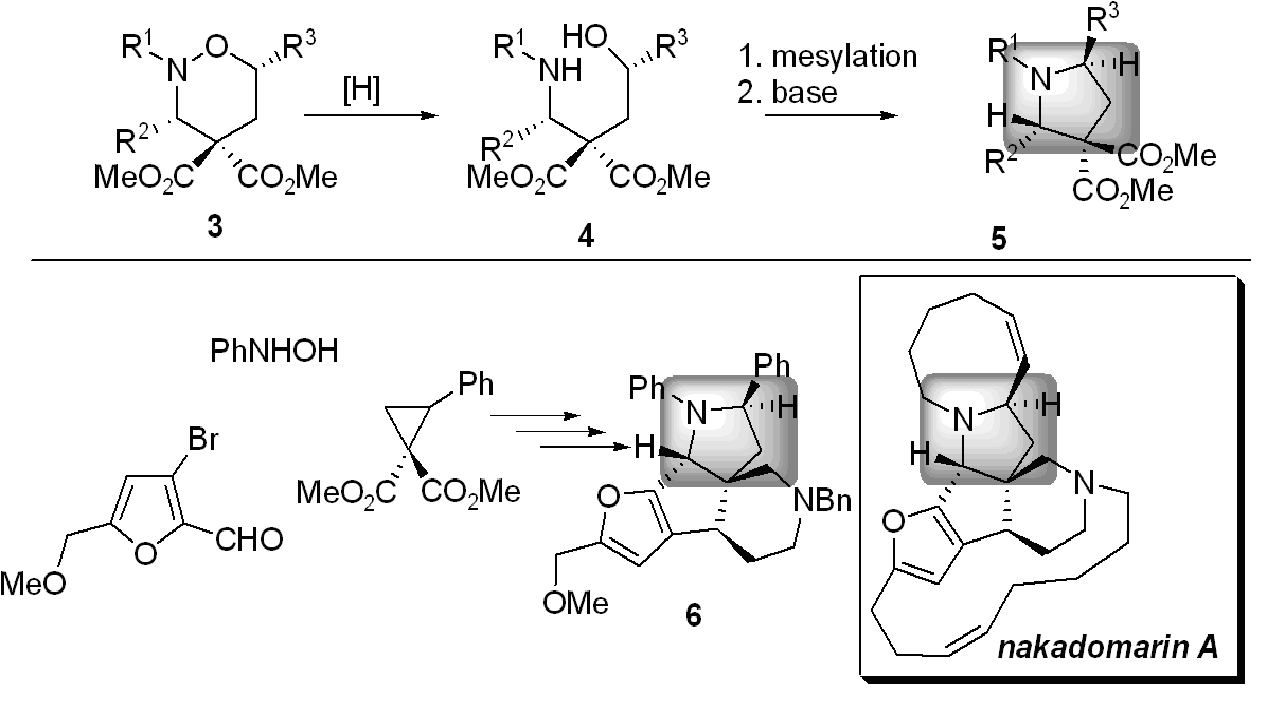
The nitrogen-oxygen bond in 3 was reduced to a 1,4-aminoalcohol 4 and converted to pyrrolidine 5 using traditional methods (Scheme 2). Notice now that the groups R2 and R3 are on opposite sides of the pyrroldine ring. A pyrrolidine with the exactly this substitution pattern (shaded box in Scheme 2) just happens to be a central structural feature of the beautifully complex alkaloid nakadomarin A, a rare natural product with anticancer activity, isolated from a sea sponge. Since only tiny amounts of nakadomarin A are available from the natural source without extinction of the species, a laboratory synthesis would be valuable from the standpoint of preparation as well as full biological and medicinal evaluation. We have prepared a model 6 containing the core structure, and have recently completed the asymmetric synthesis of (+)-nakadomarin A [DOI].
Scheme 2: A stereoselective pyrrolidine synthesis
Summary:
While the synthesis of nakadomarin A is an important project in our laboratory, it is by no means the only one. We have developed numerous new and useful chemical reactions and have successfully applied them to the laboratory synthesis of bioactive naturally occurring compounds. Our synthetic methods and strategies will continue to be of interest to both the academic and pharmaceutical organizations, and the researchers in my lab will be active contributors to synthetic chemical research.