Research
Contact
Department of Chemistry
Office: Chemistry Building Laboratory 222
Western University
London, ON, CANADA
N6A 5B7
Tel: 519-661-2111x86349
Fax: 519-661-3022
Email: Robert.Hudson
Other Research Interests
Medical Imaging
We have a long-standing interest in imaging and have worked extensively with collaborators at the Robarts Research Institute focused on the synthesis of materials for optical, magnetic resonance imaging and PET imaging for potential clinical applications.
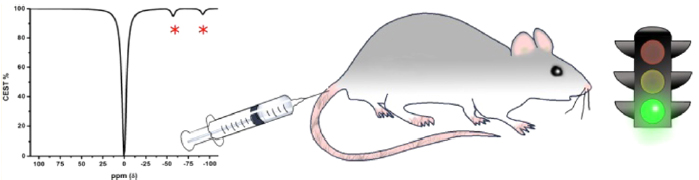
We developed peptide-decorated lanthanide metal chelators as MRI contrast agents that operate on the basis of paramagnetic chemical exchange saturation transfer (PARACEST). These ligands are similar to the well-known DOTA and DOTAM ligands.
Filip Wojciechowski, Mojmir Suchy, Alex X. Li, Hassan Azab, Robert Bartha and Robert H.E. Hudson† “A Robust and Convergent Synthesis of Dipeptide-Cyclen Conjugates as Chelators for Lanthanide Ions: New Paracest MRI Agents” Bioconjugate Chemistry, 2007, 18, 1625-1636.
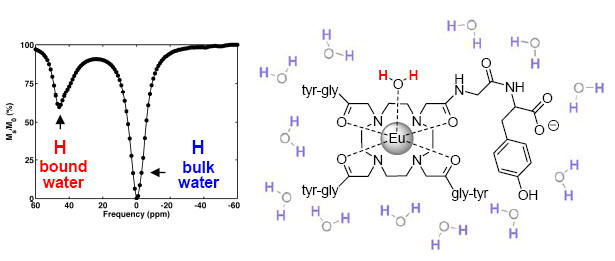
A PARACEST spectrum: the bound water signal is separated from the bulk water signal. The intense signal, approx. 45% under the experimental conditions and at physiologic pH and temperature may be used to generate contrast in PARACEST-based MR imaging.
Mojmír Suchý,Alex X. Li, Robert Bartha and Robert H.E. Hudson† “Analogs of Eu3+ DOTAM-Gly-Phe-OH and Tm3+ DOTAM-Gly-Lys-OH: Synthesis and Magnetic Properties of Potential PARACEST MRI Contrast Agents” Bioorganic and Medicinal Chemistry, 2008, 16(11), 6156-6166.
Alex X. Li, Filip Wojciechowski, Mojmir Suchy, Robert H.E. Hudson, Ravi Menon and Robert Bartha† “A Sensitive PARACEST Contrast Agent for In-vivo Temperature MRI: Eu3+-DOTAM-Gly-Phe” Magnetic Resonance in Medicine, 2008, 59, 374-381.
Synthesis of Cystamine Modified Eu3+ DOTAM-Gly-Phe-OH: A Conjugation Ready Temperature Sensitive MRI Contrast Agent. Modification of the ligand yet retention of the most desirable PARACEST effects has been achieved.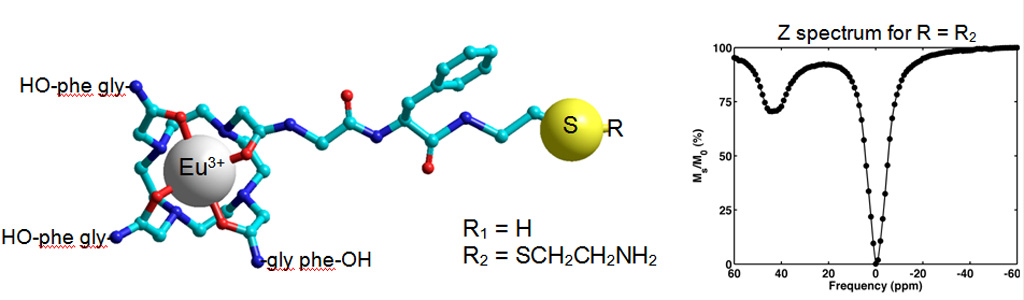
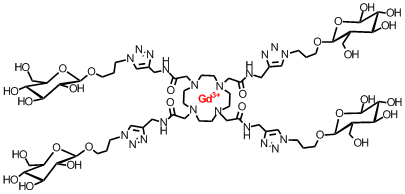
We have also extended our studies to "click" derivatization to produce ligand diversity. Where a large variety of decorated chelators may be synthesized - such as the glucose-labelled one shown below:
Mark Milne, Kirby Chicas, Alex Li, Robert Barthab and Robert H.E. Hudson† “ParaCEST MRI Contrast Agents Capable of Derivatization via “Click” Chemistry” Organic and Biomolecular Chemistry, 2012, 10(2), 287-292.
Mojmír Suchý, Robert Bartha and Robert H. E. Hudson† “Click” chemistry toward bis(DOTA-derived) heterometallic complexes: potential bimodal MRI/PET(SPECT) molecular imaging probes” RSC Advances, 2013, 3(10), 3249-3259.
Mojmír Suchý, Mark Milne, Adam A. H. Elmehriki, Nevin McVicar, Robert Bartha and Robert H. E. Hudson† “Decreasing the overall positive charge of Tm3+ DOTAM-alkyl complexes by introduction of terminal carboxylate groups and conjugation with Asp-OH: Implications for detection sensitivity and in vivo toxicity of PARACEST MRI contrast agents” Journal of Medicinal Chemistry, 2015, 58(16), 6516-6532.
Mojmír Suchý, Alex X. Li, Yin Liu, Qingping Feng, Robert Bartha and Robert H. E. Hudson† “Synthesis and Preliminary Evaluation of Contrast Agents for the Detection of Nitric Oxide Synthase (NOS) enzymatic activity by ParaCEST MRI”, Canadian Journal of Chemistry, 2016, 94(8), 715-722.
Todd K. Stevens, Mark Milne, Adam A. H. Elmehriki, Mojmír Suchý, Robert Bartha, Robert H. E. Hudson† “A DOTAM–based paraCEST agent favoring TSAP geometry for enhanced amide proton chemical shift dispersion and temperature sensitivity” Contrast Media and Molecular Imaging, 2013, 8(3), 289-292.
Adam A. H. Elmehriki, Mark Milne, Mojmír Suchý, Robert Bartha and Robert H. E. Hudson† “Complexes of Selected Late Period Lanthanide(III) Cations with DOTAM-Alkyl Ligands: A New Platform for the Development of PARACEST MRI Contrast Agents” Canadian Journal of Chemistry, 2013, 91(3), 211-219.
Alex X. Li, Mojmir Suchy, Robert H.E. Hudson, Ravi S. Menon, Robert Bartha† “Simultaneous In Vivo Temperature and pH Mapping Using a PARACEST-MRI Contrast Agent” Magnetic Resonance in Medicine, 2013, 70(4), 1016-1025.
Mojmír Suchý, Mark Milne, David W. Dodd, Alex X. Li, Nevin McVicar, Robert Bartha and Robert H. E. Hudson† “Mono- and Tetraalkyne Modified Ligands and Their Eu3+ Complexes: Utilizing ‘Click’ Chemistry to Expand the Scope of Conjugation Chemistry” European Journal of Organic Chemistry, 2011, 2011(32), 6532-6543.
Mark Milne and Robert H.E. Hudson† “Contrast agents possessing high temperature sensitivity” Chemical Communications, 2011, 47(32), 9194-9196.
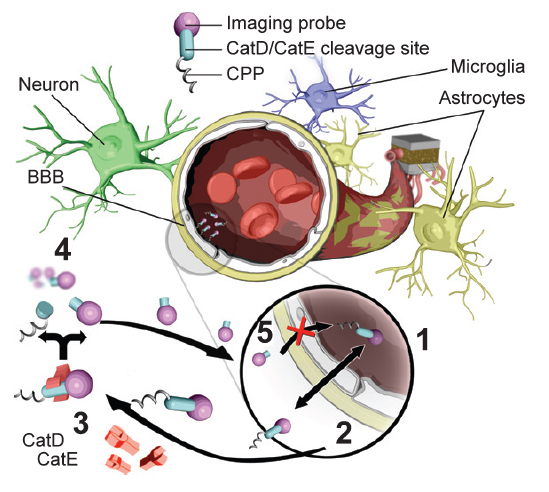
In-Vivo Fluorescence and PET imaging Probe Development
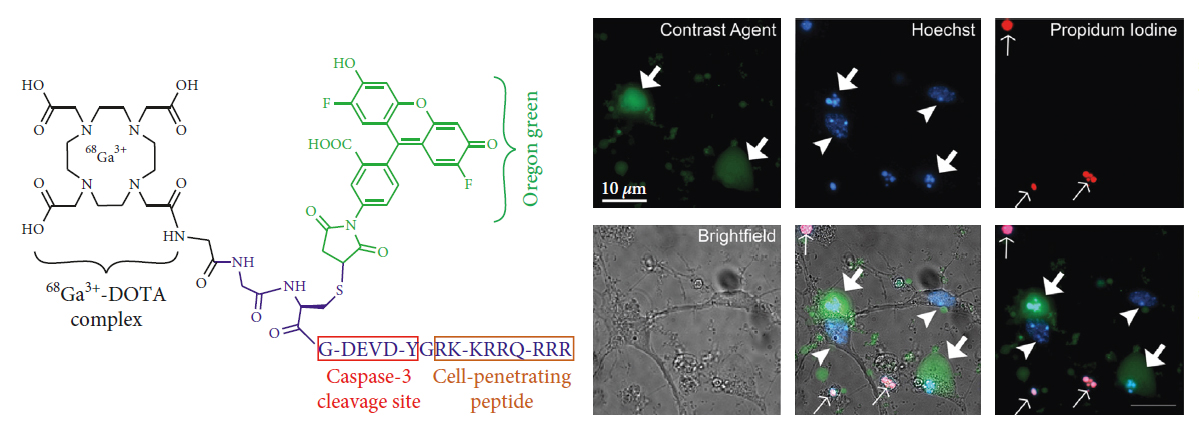
Valeriy G Ostapchenko; Jonatan Snir; Mojmir Suchy; Jue Fan; M. Rebecca Cobb; Michael Kovacs; Vania F. Prado; Robert H.E. Hudson; Stephen Pasternak; Marco Prado; Robert Bartha†, “Detection of Active Caspase-3 in Mouse Models of Stroke and Alzheimer's Disease with a Novel Dual Positron Emission Tomography and Fluorescent Contrast Agent” Contrast Media and Molecular Imaging, 2019, 6403274/1-6403274/17.
Jonatan A. Snir, Mojmir Suchy, Geron A. Bindseil, Blaine A. Chronik, Robert H. E. Hudson, Stephen H. Pasternak, Robert Bartha† “An Aspartyl Cathepsin Targeted PET Agent: Application in an Alzheimer's Disease Mouse Model" Journal of Alzheimer Disease, 2018, 61(3), 1241-1252.
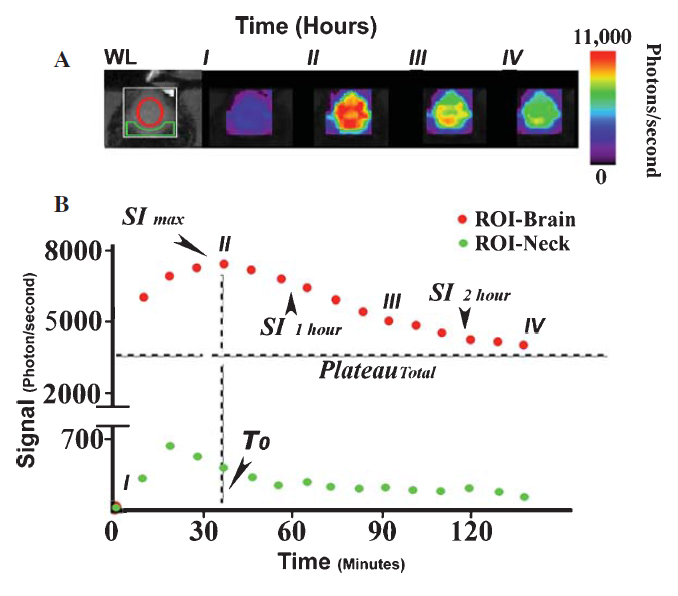
Jonatan A. Snir, Mojmir Suchy, Keith St. Lawrence, Robert H.E. Hudson, Stephen H. Pasternak and Robert Bartha† “Prolonged In-vivo Retention of a Cathepsin D Targeted Contrast Agent in Alzheimer’s Disease” Journal of Alzheimer's Disease, 2015, 48(1), 73-87.
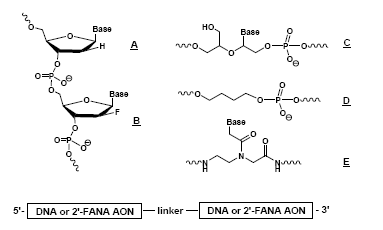
Other Applications of PNAs
Using chimeric, modified DNA/RNA and PNA to examine the structural requirements for substrate recognition of HIV RNAse H enzyme. Chemical structures used:Pt-based Anticancer Agents
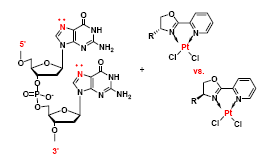
In collaboration with our Inorganic colleagues (Prof. Westcott, MTA and Prof. Jones, UWO), we have investigated the reactivity of novel cis-dichloroplatinum complexes with DNA. The archetypal compound, cisplatin (cis-diammineplatinum dichloride) is a very successful and widely used anticancer drug, but has significant side-effects and limitations - thus the search for new compounds with similar action.